All Photos(1)
About This Item
Linear Formula:
BrC6H4HNCO2C(CH3)3
CAS Number:
Molecular Weight:
272.14
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
refractive index
n20/D 1.54 (lit.)
bp
110 °C/0.3 mmHg (lit.)
density
1.342 g/mL at 25 °C (lit.)
functional group
amine
bromo
SMILES string
CC(C)(C)OC(=O)Nc1ccccc1Br
InChI
1S/C11H14BrNO2/c1-11(2,3)15-10(14)13-9-7-5-4-6-8(9)12/h4-7H,1-3H3,(H,13,14)
InChI key
UQBGKDLSIIHUEZ-UHFFFAOYSA-N
Related Categories
General description
N-(tert-Butoxycarbonyl)-2-bromoaniline, also known as tert-butyl-N-(2-bromophenyl)carbamate, is an N-Boc-protected o-bromoaniline.1 It reacts with ethyl perfluorooctanoate in the presence of tert-butyllithium to form the corresponding 1-hydroxy-1H-perfluorooctyl compound.
Application
N-(tert-Butoxycarbonyl)-2-bromoaniline may be used in the synthesis of N-boc-protected o-aryl anilines, which undergoes coupling reaction with bromoacetylenes to form the corresponding arene?ynamides.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
230.0 °F - closed cup
Flash Point(C)
110 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel reduction of perfluoroalkyl ketones with lithium alkoxides.
Sokeirik YS, et al.
Journal of Fluorine Chemistry, 127(1), 150-152 (2006)
Yousuke Yamaoka et al.
The Journal of organic chemistry, 80(2), 957-964 (2014-12-30)
A Brønsted acid-promoted arene-ynamide cyclization has been developed to construct the 3H-pyrrolo[2,3-c]quinolines. This reaction consists of the generation of a highly reactive keteniminium intermediate from arene-ynamide activated by a Brønsted acid and electrophilic aromatic substitution reaction to give arene-fused quinolines
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service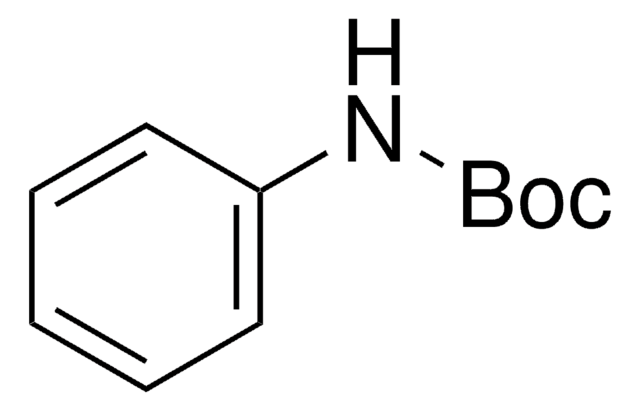
![4-[(N-Boc)aminomethyl]aniline 97%](/deepweb/assets/sigmaaldrich/product/structures/341/155/530c425c-7e6e-435e-a28a-9d40b05b938a/640/530c425c-7e6e-435e-a28a-9d40b05b938a.png)

![[4,4′-Bis(1,1-dimethylethyl)-2,2′-bipyridine] nickel (II) dichloride](/deepweb/assets/sigmaaldrich/product/structures/471/091/6faa29b1-bf8a-4d87-90b2-4cc55e082620/640/6faa29b1-bf8a-4d87-90b2-4cc55e082620.png)