All Photos(2)
About This Item
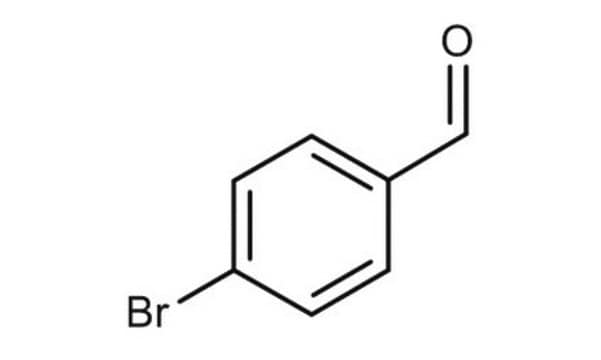
Linear Formula:
Br2C6H3CHO
CAS Number:
Molecular Weight:
263.91
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
mp
84-88 °C (lit.)
Quality Level
functional group
aldehyde
bromo
SMILES string
Brc1cc(Br)cc(C=O)c1
InChI
1S/C7H4Br2O/c8-6-1-5(4-10)2-7(9)3-6/h1-4H
InChI key
ZLDMZIXUGCGKMB-UHFFFAOYSA-N
Related Categories
Application
Reactant involved in:
- Suzuki-Miyaura cross-coupling reactions
- Synthesis of blue fluorescent dye derivatives for organic light emitting diodes
- Sharpless kinetic resolution for the formation of Baylis-Hillman enal adducts
- Synthesis of podophyllotoxin mimetic pyridopyrazoles as anticancer agents
- Allylic alkylation
- Synthesis of C2-symmetric biphosphine ligand I
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Coordination-Driven Self-Assembly of Fullerene-Functionalized Pt (II) Metallacycles.
Neti VSPK, et al.
Organometallics, 34(20), 4813-4815 (2015)
Synthesis and luminescence characteristics of conjugated dendrimers with 2, 4, 6-triaryl-1, 3, 5-triazine periphery.
Kim CK, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 44(1), 254-263 (2006)
Conformational Behavior of Conjugated Polymers With Oligo (phenylene vinylene) Side Chains.
Peeter H and Koeckelberghs G.
Macromolecular Chemistry and Physics, 214(5), 538-546 (2013)
Punit P Seth et al.
Bioorganic & medicinal chemistry letters, 14(22), 5569-5572 (2004-10-16)
The preparation and evaluation of novel aryl urea analogs as broad-spectrum antibacterial agents is described. Numerous compounds showed low micromolar minimum inhibitory concentrations (MIC) against both Gram-positive and Gram-negative bacteria. Selected analogs also exhibited in vivo efficacy in a lethal
Synthesis, crystal structures, and antibacterial activity of a series of hydrazone compounds derived from 4-methylbenzohydrazide.
Lei Y, et al.
Journal of the Chilean Chemical Society, 60(2), 2961-2965 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service