47749
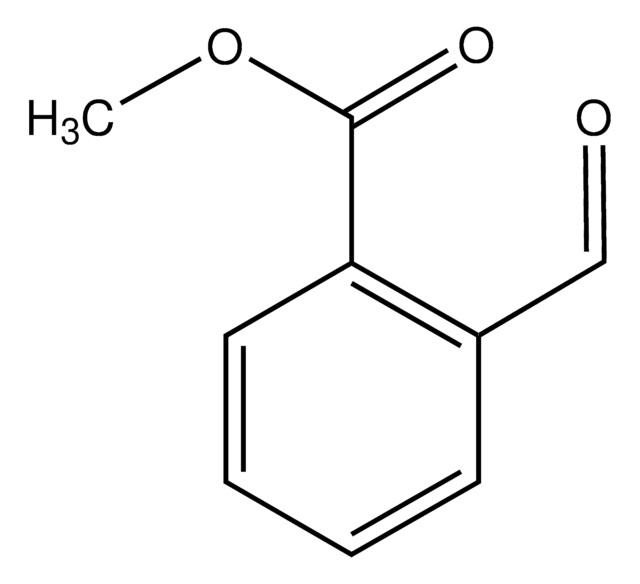
Methyl 3-formylbenzoate
≥98.0% (GC)
Synonym(s):
Methyl benzaldehyde-3-carboxylate
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H8O3
CAS Number:
Molecular Weight:
164.16
Beilstein:
2690668
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98.0% (GC)
form
solid
mp
48-52 °C
functional group
aldehyde
ester
SMILES string
COC(=O)c1cccc(C=O)c1
InChI
1S/C9H8O3/c1-12-9(11)8-4-2-3-7(5-8)6-10/h2-6H,1H3
InChI key
UVSBCUAQEZINCQ-UHFFFAOYSA-N
Application
Methyl 3-formylbenzoate may be used in the preparation of the following bioactive compounds:
- meso-Tetrakis(3-carboxyphenyl)porphyrin via condensation with pyrrole.
- Methyl 3-[4-(1-methyl-3-phenylureido)phenylaminomethyl]benzoate, which shows moderate SENP1 protease inhibition activity.
- An N-methyl-sulfonylhydrazone derivative for use as anti-diabetic agent with good plasma stability.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Discovery of 1-[4-(N-benzylamino) phenyl]-3-phenylurea derivatives as non-peptidic selective SUMO-sentrin specific protease (SENP) 1 inhibitors.
Uno M, et al.
Bioorganic & Medicinal Chemistry Letters, 22(16), 5169-5173 (2012)
Tetraphilin: a four-helix proton channel built on a tetraphenylporphyrin framework.
Akerfeldt KS, et al.
Journal of the American Chemical Society, 114(24), 9656-9657 (1992)
Synthesis, solubility, plasma stability, and pharmacological evaluation of novel sulfonylhydrazones designed as anti-diabetic agents.
Zapata-Sudo G, et al.
Drug design, development and therapy, 10, 2869-2869 (2016)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service