322377
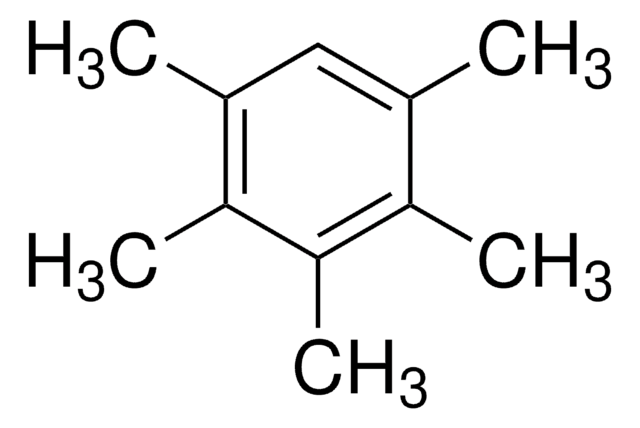
Hexamethylbenzene
99%
Synonym(s):
1,2,3,4,5,6-Hexamethylbenzene, Mellitene
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
C6(CH3)6
CAS Number:
Molecular Weight:
162.27
Beilstein:
1905834
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
bp
264 °C (lit.)
mp
164-166 °C (lit.)
SMILES string
Cc1c(C)c(C)c(C)c(C)c1C
InChI
1S/C12H18/c1-7-8(2)10(4)12(6)11(5)9(7)3/h1-6H3
InChI key
YUWFEBAXEOLKSG-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Hexamethylbenzene, also known as Mellitene, is an arene ligand that is commonly used as a precursor in the synthesis of organometallic complex.
Application
- Ru(II)-Arene Complexes of Curcumin and Bisdesmethoxycurcumin Metabolites: Investigates the structure and reactivity of Ru(II)-arene complexes with curcumin metabolites, shedding light on potential applications in drug development and synthesis pathways involving hexamethylbenzene derivatives (Dyson et al., 2024).
- Theoretical study on degradation of polymethyl substituted benzenes by OH radicals in the atmosphere: Offers a comprehensive computational analysis on the atmospheric reactions of polymethyl substituted benzenes, including hexamethylbenzene, with OH radicals, relevant for understanding their environmental impacts (Zhao et al., 2024).
- Revisiting the bonding of the pentagonal-pyramidal C(6)H(6)(2+) and C(6)(CH(3))(6)(2+) dications: Explores the unique bonding and structural characteristics of hexamethylbenzene in dicationic forms, providing insights into its chemical behavior under various electronic conditions (Torres et al., 2023).
- Naphthalene Diimide-Functionalized Half-Sandwich Ru(II) Complexes as Mitochondria-Targeted Anticancer and Antimetastatic Agents: Discusses the synthesis and biological evaluation of Ru(II) complexes involving hexamethylbenzene structures, aimed at developing new therapeutic agents (Yang et al., 2023).
- Comparative Study on Carbon Erosion of Nickel Alloys in the Presence of Organic Compounds under Various Reaction Conditions: Examines the impact of organic compounds, including hexamethylbenzene, on the wear and corrosion of nickel alloys, relevant for industrial applications (Volodin et al., 2022).
Hexamethylbenzene is used as a reagent during the synthesis of ketodiepoxides via hydroxylation reactions.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
The pentagonal-pyramidal hexamethylbenzene dication: many shades of coordination chemistry at carbon
Johannes K EMN, et al.
Chemistry?A European Journal , 24, 12340-12345 (2018)
Effects of Substituted Triarylphosphine Ligands on Electron Transfer in [(p-cymene) Ru] Complexes
Anthony M N, et al.
Organometallics (2024)
Applications of Rozen?s Reagent in Oxygen-Transfer and C--H Activation Reactions
Kuldeep S, et al.
Synthesis, 51, 371-383 (2019)
P D Gorycki et al.
Chemical research in toxicology, 7(6), 745-751 (1994-11-01)
Hexamethyl(Dewar benzene) (HMDB) was selected to probe the intermediacy of an alkene radical cation during cytochrome P450 (P450) catalyzed epoxidation. P450 catalyzed the allylic oxidation of HMDB exclusively; no rearranged products (indicative of a radical cation intermediate) nor epoxide was
Yaw Kai Yan et al.
Journal of biological inorganic chemistry : JBIC : a publication of the Society of Biological Inorganic Chemistry, 11(4), 483-488 (2006-04-11)
Ruthenium(II) arene anticancer complexes [(eta6-arene)Ru(en)Cl]PF6 (arene is hexamethylbenzene, p-cymene, indan; en is ethylenediamine) can catalyse regioselective reduction of NAD+ by formate in water to form 1,4-NADH, at pD 7.2, 37 degrees C, and in the presence of air. The catalytic
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service