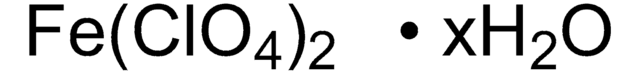278904
Potassium dioxide
powder
Synonym(s):
Potassium superoxide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
KO2
CAS Number:
Molecular Weight:
71.10
EC Number:
MDL number:
UNSPSC Code:
12352303
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
form
powder
Quality Level
SMILES string
[K+].[O][O-]
InChI
1S/K.HO2/c;1-2/h;1H/q+1;/p-1
InChI key
DUPSQGGNCHNYTQ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Ox. Sol. 1 - Skin Corr. 1B
Supplementary Hazards
Storage Class Code
5.1A - Strongly oxidizing hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Young Sun Yun et al.
Journal of biochemistry and molecular biology, 36(3), 282-287 (2003-06-06)
The production of superoxide dismutase (SOD) varied in Deinococcus radiophilus, the UV resistant bacterium, depending upon different phases of growth, UV irradiation, and superoxide treatment. A gradual increase in total SOD activity occurred up to the stationary phases. The electrophoretic
Andreas Daiber et al.
The Journal of biological chemistry, 277(14), 11882-11888 (2002-01-24)
Based on the previous report of McCord and co-workers (Crow, J. P., Beckman, J. S., and McCord, J. M. (1995) Biochemistry 34, 3544-3552), the zinc dithiolate active site of alcohol dehydrogenase (ADH) has been studied as a target for cellular
N Wada et al.
Journal of bioluminescence and chemiluminescence, 12(1), 15-20 (1997-01-01)
Addition of KO2 in dimethyl sulfoxide (DMSO) to the in vitro bacterial luciferase reaction subsequent to its initiation resulted in a biphasic decay of light emission. The first and more rapid phase is attributed to quenching by DMSO. With DMSO
Weiqin Jiang et al.
Organic letters, 5(1), 43-46 (2003-01-03)
Potassium superoxide was examined as an alternative oxidation reagent for the Winterfeldt reaction. KO(2) was found to be superior to the original Winterfeldt protocol for base-sensitive substrates. [reaction--see text]
Brant A Smith et al.
Environmental science & technology, 38(20), 5465-5469 (2004-11-17)
The reactive oxygen species responsible for the transformation of carbon tetrachloride (tetrachloromethane, CT) by modified Fenton's reagent using hydrogen peroxide (H2O2) concentrations >0.1 M was investigated. Addition of the hydroxyl radical scavenger 2-propanol to modified Fenton's reactions did not significantly
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service