All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C9H9N
CAS Number:
Molecular Weight:
131.17
Beilstein:
111026
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥97%
form
liquid
refractive index
n20/D 1.606 (lit.)
bp
133 °C/26 mmHg (lit.)
density
1.051 g/mL at 20 °C (lit.)
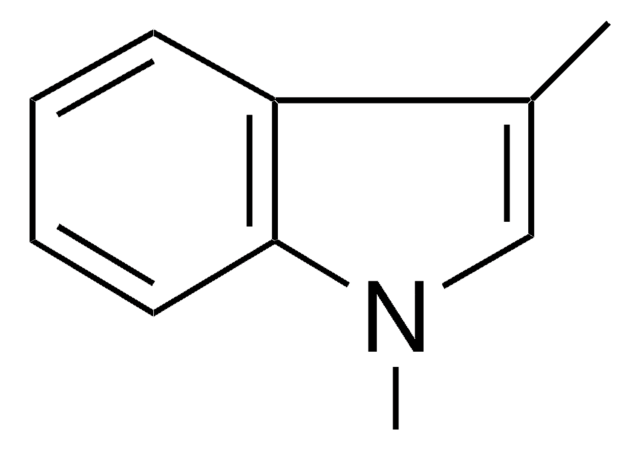
SMILES string
Cn1ccc2ccccc12
InChI
1S/C9H9N/c1-10-7-6-8-4-2-3-5-9(8)10/h2-7H,1H3
InChI key
BLRHMMGNCXNXJL-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1-Methylindole undergoes Au(III)/TPPMS-catalyzed benzylation reaction with benzhydryl and benzylic alcohols.
Application
1-Methylindole was used in the determination of association constant for the electron-donor-acceptor complexes of 1-methylindole with 1-(2,4,6-trinitrophenyl) propan-2-one.
Reactant for preparation of:
- Pharmaceutically active 2-oxo-1-pyrrolidine analogues
- Non-receptor tyrosine kinase (Src kinase) inhibitors
- PET agents for imaging of protein kinase C (PKC)
- Ynediones as highly reactive Michael systems
- Anticancer agents
- Polycyclic derivatives of indoles
- PET agents for imaging of glycogen synthase kinase-3 (GSK-3)
- Anti-prion disease agents
- Bisindole derivatives with antihyperlipidemic activity
- PET cancer imaging agents
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Benjamin S Lane et al.
Journal of the American Chemical Society, 127(22), 8050-8057 (2005-06-02)
We have recently developed palladium-catalyzed methods for direct arylation of indoles (and other azoles) wherein high C-2 selectivity was observed for both free (NH)-indole and (NR)-indole. To provide a rationale for the observed selectivity ("nonelectrophilic" regioselectivity), mechanistic studies were conducted
Nicholas R Deprez et al.
Journal of the American Chemical Society, 128(15), 4972-4973 (2006-04-13)
This communication describes the rational development of a PdII-catalyzed method for the direct 2-arylation of indoles using [Ar-IIII-Ar]BF4. These reactions proceed under remarkably mild conditions (often at room temperature and in the presence of ambient air and moisture), and these
Martin G Banwell et al.
Organic letters, 8(21), 4959-4961 (2006-10-06)
[reaction: see text] Reaction of N-methylindole (4) with 6,6-dibromobicyclo[3.1.0]hexane (5) in the presence of silver tetrafluoroborate affords conjugate 7 in 67% yield. This product can be readily elaborated to compounds 12b and 13b which embody the polycyclic frameworks associated with
Hongmei Liu et al.
Malaria journal, 17(1), 348-348 (2018-10-07)
Anopheles sinensis is an important vector for the spread of malaria in China. Olfactory-related behaviours, particularly oviposition site seeking, offer opportunities for disrupting the disease-transmission process. This is the first report of the identification and characterization of AsinOrco and AsinOR10
Association constants for the electron-donor-acceptor complexes of indole and 1-methylindole with 1-(2, 4, 6-trinitrophenyl) propan-2-one from nuclear magnetic resonance shift measurements. An anomalous scatchard plot.
Chudek JA, et al.
J. Chem. Soc., Faraday, 84(4), 1145-1152 (1988)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service