39319
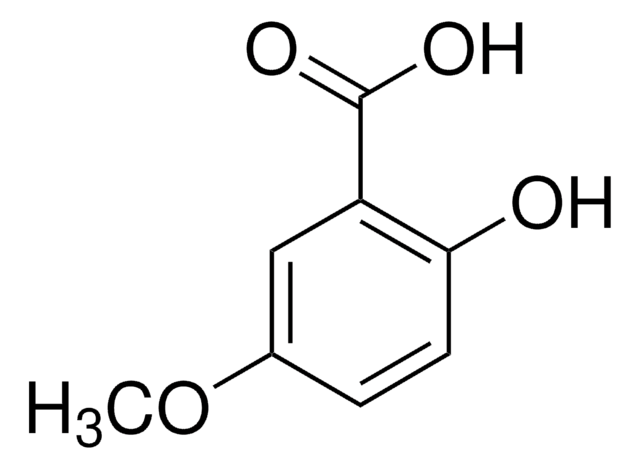
2,5-Dihydroxybenzoic acid
matrix substance for MALDI-MS, ≥99.5% (HPLC), Ultra pure
Synonym(s):
2,5-DHBA, DHB, Gentisic acid, Hydroquinonecarboxylic acid
About This Item
Recommended Products
grade
matrix substance for MALDI-MS
Quality Level
Assay
≥99.5% (HPLC)
analyte functional class(es)
polymers
analyte chemical class(es)
glycans, lipids, organic molecules, peptides, proteins
technique(s)
MALDI-MS: suitable
mp
203-207 °C
204-208 °C (lit.)
solubility
methanol: 10 mg/mL, clear
cation traces
Al: ≤1 mg/kg
Ba: ≤1 mg/kg
Ca: ≤1 mg/kg
Cd: ≤1 mg/kg
Co: ≤1 mg/kg
Cr: ≤1 mg/kg
Cu: ≤1 mg/kg
Fe: ≤1 mg/kg
K: ≤1 mg/kg
Li: ≤1 mg/kg
Mg: ≤1 mg/kg
Mn: ≤1 mg/kg
Na: ≤1 mg/kg
Ni: ≤1 mg/kg
Pb: ≤1 mg/kg
Sr: ≤1 mg/kg
Zn: ≤1 mg/kg
SMILES string
OC(=O)c1cc(O)ccc1O
InChI
1S/C7H6O4/c8-4-1-2-6(9)5(3-4)7(10)11/h1-3,8-9H,(H,10,11)
InChI key
WXTMDXOMEHJXQO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Quantitative MALDI-MS and Imaging of Fungicide Pyrimethanil in Strawberries with 2-Nitrophloroglucinol as an Effective Matrix.: This research highlights the application of 2,5-Dihydroxybenzoic acid (DHB) as an effective matrix for MALDI-MS imaging, providing enhanced detection sensitivity for fungicides in agricultural samples. The study showcases DHB′s role in improving the quantification and spatial resolution of pesticide residues within fruit matrices, demonstrating its utility in food safety and chemical analysis (Liang et al., 2024).
- A maize enzyme from the 2-oxoglutarate-dependent oxygenase family with unique kinetic properties, mediates resistance against pathogens and regulates senescence.: Utilizing 2,5-Dihydroxybenzoic acid in enzyme kinetics studies, this paper reveals its critical role in modulating plant defense mechanisms against pathogens. The study provides insights into the biochemical pathways influenced by DHB, underscoring its importance in plant biochemistry and resistance breeding (Casati et al., 2024).
- Unveiling the distribution of free and bound phenolic acids, flavonoids, anthocyanins, and proanthocyanidins in pigmented and non-pigmented rice genotypes.: This comprehensive analysis employs 2,5-Dihydroxybenzoic acid to investigate the phenolic content in different rice varieties, highlighting its role in enhancing the detection of health-beneficial compounds. The research supports DHB′s application in nutritional studies and crop quality assessments (Thulasinathan et al., 2024).
- Novel Small Molecule Matrix Screening for Simultaneous MALDI Mass Spectrometry Imaging of Multiple Lipids and Phytohormones.: Leveraging the chemical properties of 2,5-Dihydroxybenzoic acid, this study introduces a novel matrix for MALDI-MS imaging, enhancing the simultaneous visualization of lipids and phytohormones in biological tissues. It highlights the versatility of DHB in facilitating complex biochemical analyses (Shi et al., 2024).
- 2-Hydroxy-5-nitro-3-(trifluoromethyl)pyridine as a Novel Matrix for Enhanced MALDI Imaging of Tissue Metabolites.: This research demonstrates the use of DHB-based matrices to improve the MALDI imaging of metabolites, offering greater sensitivity and specificity in clinical diagnostics and metabolic studies. The findings contribute to the broader application of DHB in analytical chemistry (Wang et al., 2024).
Packaging
related product
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
One of the most important aspects of our ultra-pure MALDI matrix substances is their ability to dissolve rapidly and completely; a brief vortex mixing is typically sufficient.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service