All Photos(1)
About This Item
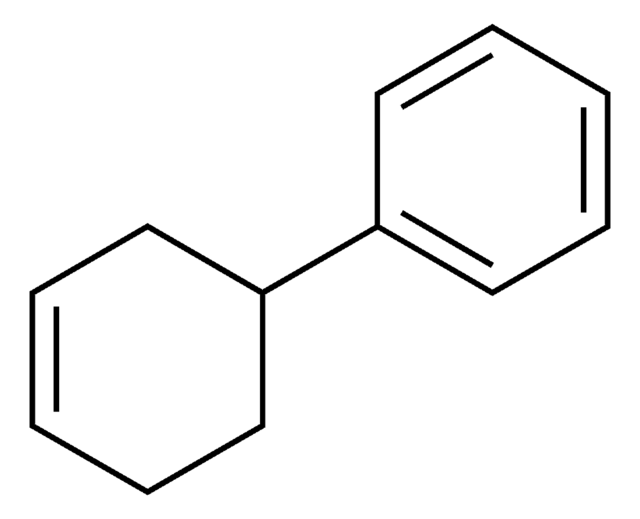
Linear Formula:
C6H5C6H9
CAS Number:
Molecular Weight:
158.24
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
refractive index
n20/D 1.57 (lit.)
bp
251-253 °C (lit.)
mp
−11 °C (lit.)
density
0.994 g/mL at 25 °C (lit.)
SMILES string
C1CCC(=CC1)c2ccccc2
InChI
1S/C12H14/c1-3-7-11(8-4-1)12-9-5-2-6-10-12/h1,3-4,7-9H,2,5-6,10H2
InChI key
WCMSFBRREKZZFL-UHFFFAOYSA-N
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
217.4 °F - closed cup
Flash Point(C)
103.00 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
D H Young et al.
Bioorganic & medicinal chemistry letters, 11(11), 1393-1396 (2001-05-30)
Phenylcyclohexenes (PCHs) [e.g., trans-4-nitro-5-(2,3,4-trimethoxyphenyl)cyclohexene, 2d] were found to bind weakly to the colchicine site of bovine tubulin, but are the first mimics of colchicine found to have high activity towards plant cells. Structure-activity relationships for PCHs and biphenyl AC-ring analogues
R L Beard et al.
Bioorganic & medicinal chemistry letters, 11(6), 765-768 (2001-03-30)
Retinoids are natural and synthetic analogues of the hormone retinoic acid. Systemic retinoid agonist therapy is usually associated with toxic side effects, such as mucocutaneous toxicity, which may be alleviated by the use of topical retinoid antagonists. We report the
S Chakrabarti et al.
Toxicology and applied pharmacology, 69(2), 179-184 (1983-06-30)
The metabolic disposition of 1-[14C]phenylcyclohexene ([14C]PC) was examined in rats after ip or iv drug administration. Radioactivity, which was accumulated by various organs, peaked within 30 min after ip administration of [14C]PC (0.21 mg/kg). A significant amount of this radioactivity
B R Martin et al.
Drug metabolism and disposition: the biological fate of chemicals, 10(6), 685-689 (1982-11-01)
The in vitro metabolism of 1-3H-phenyl-1-cyclohexene (3H-PC) was studied in a crude microsomal preparation from mouse livers. The major routes of metabolism were allylic hydroxylation, oxidation of the allylic alcohol, and epoxidation-hydrolysis. The following metabolites were identified by comparison with
A S Freeman et al.
Journal of pharmaceutical sciences, 70(9), 1002-1004 (1981-09-01)
Parsley cigarettes containing [3H]phencyclidine were machine smoked, and the mainstream smoke was trapped in glass wool filters. Radioactivity was extracted from these filters with chloroform. The average recoveries of radioactivity were 76, 85, 70, and 69% for cigarettes containing 3
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service