D18609
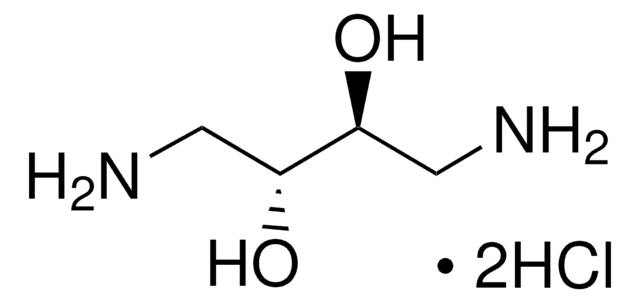
1,3-Diamino-2-propanol
95%
Synonym(s):
2-Hydroxy-1,3-propanediamine, 1,3-Diamino-2-hydroxypropane
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2CH2CH(OH)CH2NH2
CAS Number:
Molecular Weight:
90.12
Beilstein:
741859
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
solid
mp
40-44 °C (lit.)
SMILES string
NCC(O)CN
InChI
1S/C3H10N2O/c4-1-3(6)2-5/h3,6H,1-2,4-5H2
InChI key
UYBWIEGTWASWSR-UHFFFAOYSA-N
Gene Information
rat ... Grin2a(24409)
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- 1,3-Diamino-2-propanol is a versatile bidentate diamine ligand which is used in the synthesis of a variety of organometallic compounds.
- It is a precursor to synthesize the fluorogenic dsDNA binder, N1,N3-bis(4-amidinophenyl)propane-1,3-diamine (BAPPA).
- It can also be used as a branching unit in the synthesis of peptide dendrimers.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bis-4-aminobenzamidines: versatile, fluorogenic A/T-selective dsDNA binders.
Vazquez O, et al.
Organic Letters, 12(2), 216-219 (2009)
Efficient Phosphodiester Binding and Cleavage by a ZnII Complex Combining Hydrogen?Bonding Interactions and Double Lewis Acid Activation.
Feng G, et al.
Angewandte Chemie (International Edition in English), 45(42), 7056-7059 (2006)
Syntheses, structures, and electrochemical properties of inclusion compounds of cucurbit [8] uril with cobalt (III) and nickel (II) complexes.
Mitkina T V, et al.
Inorganic Chemistry, 47(15), 6748-6755 (2008)
Synthesis and esterolytic activity of catalytic peptide dendrimers.
Lagnoux D, et al.
Chemistry?A European Journal , 10(5), 1215-1226 (2004)
L Alhonen-Hongisto
The Biochemical journal, 190(3), 747-754 (1980-09-15)
1. The mechanism of stimulation of S-adenosylmethionine decarboxylase (EC 4.1.1.50) activity by inhibitors of ornithine decarboxylase (EC 4.1.1.17), namely dl-alpha-difluoromethylornithine, 1,3-diaminopropane and 1,3-diaminopropan-2-ol, was studied in Ehrlich ascites-tumour cells grown in suspension cultures. 2. Difluoromethylornithine and diaminopropane, although decreasing the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service