913715
KB05-SLF
Synonym(s):
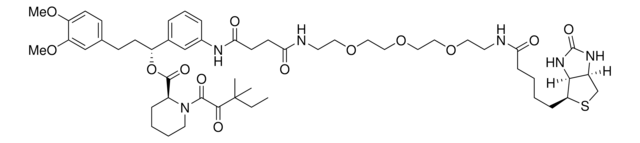
(R)-1-(3-(1-(4-(N-(4-Bromophenyl)acrylamido)phenyl)-1-oxo-5,8,11-trioxa-2-azatetradecan-14-amido)phenyl)-3-(3,4-dimethoxyphenyl)propyl (S)-1-(3,3-dimethyl-2-oxopentanoyl)piperidine-2-carboxylate, Electrophilic PROTAC®, Heterobifunctional conjugate for E3 ubiquitin ligase discovery
About This Item
Recommended Products
ligand
electrophilic fragment
Quality Level
form
powder
storage temp.
−20°C
SMILES string
O=C(C=C)N(C1=CC=C(C(NCCOCCOCCOCCC(NC2=CC=CC([C@H](OC([C@@H]3CCCCN3C(C(C(C)(C)CC)=O)=O)=O)CCC4=CC(OC)=C(OC)C=C4)=C2)=O)=O)C=C1)C5=CC=C(Br)C=C5
Application
This proteomic approach to E3 discovery was demonstrated by Zhang et al in the discovery that DCAF16 mediated nuclear FKBP12 degradation via KB02-SLF.
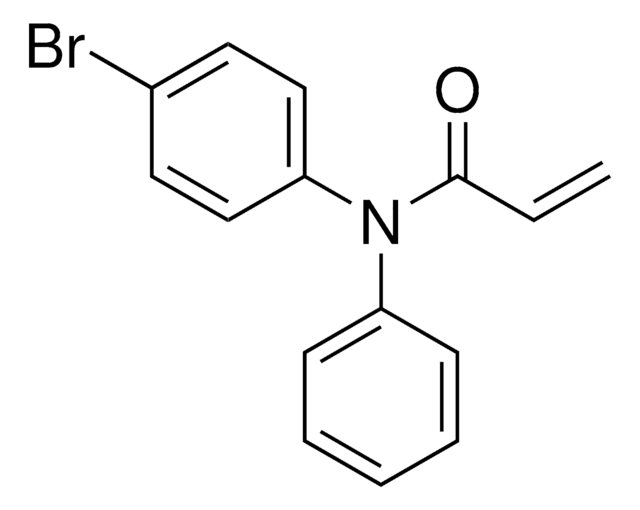
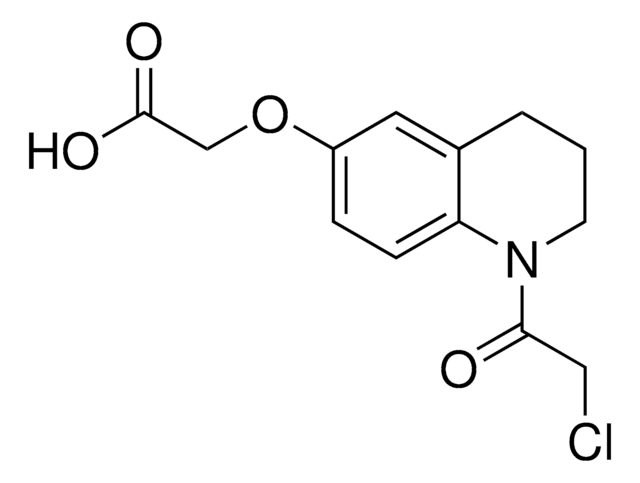
Additional electrophilic PROTACs were developed incorporating scout fragments with broad cysteine reactivity:
- KB02-SLF (914738) containing chloroacetamide scout fragment 912131
- KB03-SLF (914975) containing chloroacetamide scout fragment 912654
Related tools:
- Additional bifunctional tools for FKPB12 variants: dTAG-13 (SML2601 for FKBP12F36V) and Biotin-SLF (914223 for FKBP12)
- Inhibitors useful in validation of proteasomal-mediated degradation: MG123 (SML1135) and MLN4924 (5.05477 for Cullin-RING ubiquitin ligases that regulate neddylation of Cullin proteins)
- Cereblon (CRBN) affinity probe: Biotin-Thalidomide (913979)
Other Notes
Technology Spotlight: Proteomic Ligandability Assessment
Technology Spotlight: Building PROTAC® Degraders for Targeted Protein Degradation
Legal Information
related product
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service