All Photos(2)
About This Item
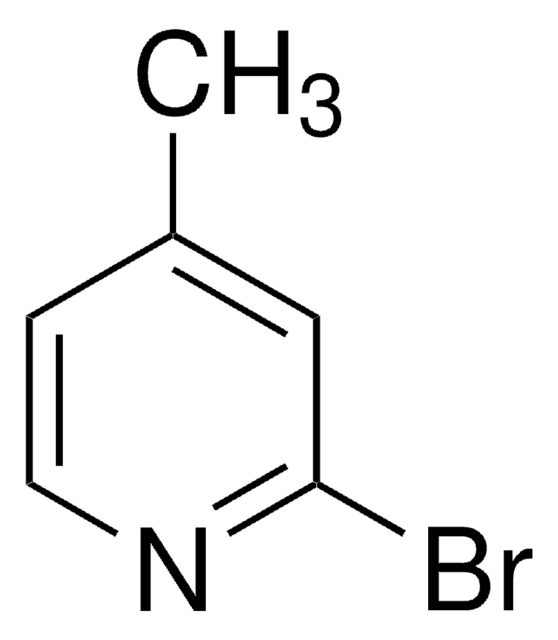
Empirical Formula (Hill Notation):
C6H6BrN
CAS Number:
Molecular Weight:
172.02
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
refractive index
n20/D 1.568 (lit.)
bp
218-219 °C (lit.)
density
1.544 g/mL at 25 °C (lit.)
functional group
bromo
SMILES string
Cc1cccnc1Br
InChI
1S/C6H6BrN/c1-5-3-2-4-8-6(5)7/h2-4H,1H3
InChI key
PZSISEFPCYMBDL-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lynne H Thomas et al.
Acta crystallographica. Section C, Crystal structure communications, 69(Pt 11), 1279-1288 (2013-11-07)
Controlled introduction of proton transfer into the design of a series of molecular complexes is described, delivering the systematic production of ionic molecular complexes (molecular salts). The controlled production of molecular salts has relevance as a potential strategy in the
Copper (ii) complexes of 2-halo-3-methylpyridine: synthesis, structure, and magnetic behaviour of Cu (2-X-3-CH 3 py) 2 X' 2 [X, X'= chlorine or bromine; py= pyridine].
Herringer SN, et al.
Dalton Transactions, 40(16), 4242-4252 (2011)
Synthesis of 2, 2'-Bipyridines: Versatile Building Blocks for Sexy Architectures and Functional Nanomaterials.
European Journal of Organic Chemistry, 2, 235-254 (2004)
Alan C Spivey et al.
Organic letters, 9(5), 891-894 (2007-02-10)
[reaction: see text] The scope and limitations of the conjugate addition of 2- and the first 4-pyridyl Gilman homocuprates to various alpha,beta-unsaturated Michael acceptors are delineated. The conjugate addition of the cuprate of 2-bromo-3-methylpyridine to (E)-methyl crotonate then diastereoselective enolate
Kelnner W R de França et al.
The Journal of organic chemistry, 67(6), 1838-1842 (2002-03-16)
2,2'-Bipyridine (bpy) and a series of dimethyl-2,2'-bipyridines were synthesized from 2-bromopyridine and 2-bromomethylpyridines, respectively, using an electrochemical process catalyzed by nickel complexes. The method is simple and efficient, with isolated yields between 58 and 98% according to the structure. We
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service