All Photos(1)
About This Item
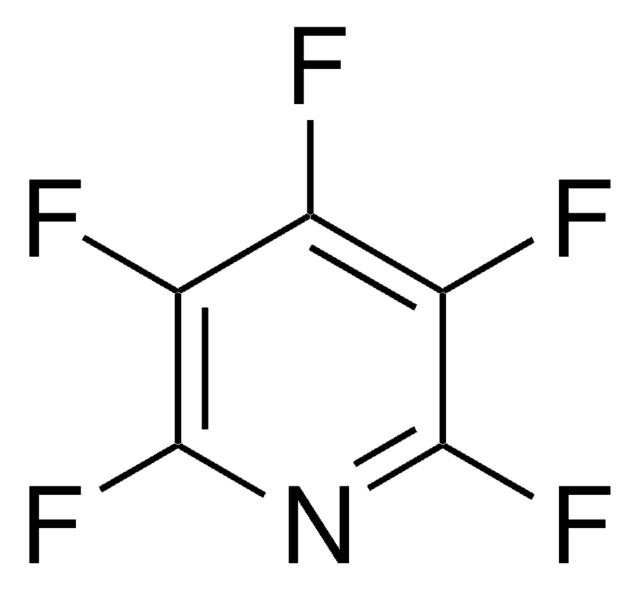
Empirical Formula (Hill Notation):
C6H3F3
CAS Number:
Molecular Weight:
132.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥98%
form
liquid
refractive index
n20/D 1.423 (lit.)
bp
94-95 °C (lit.)
density
1.28 g/mL at 25 °C (lit.)
SMILES string
Fc1cccc(F)c1F
InChI
1S/C6H3F3/c7-4-2-1-3-5(8)6(4)9/h1-3H
InChI key
AJKNNUJQFALRIK-UHFFFAOYSA-N
General description
Crystal structure 1,2,3-trifluorobenzene was reported. The microwave spectrum of 1,2,3-trifluorobenzene was studied using a molecular beam Fourier transform microwave spectrometer. Laser-induced fluorescence spectra of the radical cation of 1,2,3-trifluorobenzene was determined in both the gas phase and solid Ne matrices.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
24.8 °F - closed cup
Flash Point(C)
-4 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave Spectrum of 1, 2, 3-Trifluorobenzene.
Onda M, et al.
Journal of Molecular Spectroscopy, 169(2), 480-483 (1995)
The laser-induced fluorescence spectrum of the 1, 2, 3-trifluorobenzene radical cation.
Bondybey VE, et al.
Journal of Molecular Spectroscopy, 84(1), 124-131 (1980)
Michael T Kirchner et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 11), o2670-o2670 (2009-01-01)
In the title compound, C(6)H(3)F(3), weak electrostatic and dispersive forces between C(δ+)-F(δ-) and H(δ+)-C(δ-) groups are at the borderline of the hydrogen-bond phenomenon and are poorly directional and further deformed in the presence of π-π stacking inter-actions. The mol-ecule lies
Marissa A Dobulis et al.
The Journal of chemical physics, 152(20), 204309-204309 (2020-06-04)
The broadband photoelectron source realized by detaching O2-·X (X = neutral unsaturated molecule) complexes offers a unique opportunity to probe temporary anion states of the unsaturated species. Detachment of the ion molecule complex typically accesses a dissociative portion of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service