All Photos(1)
About This Item
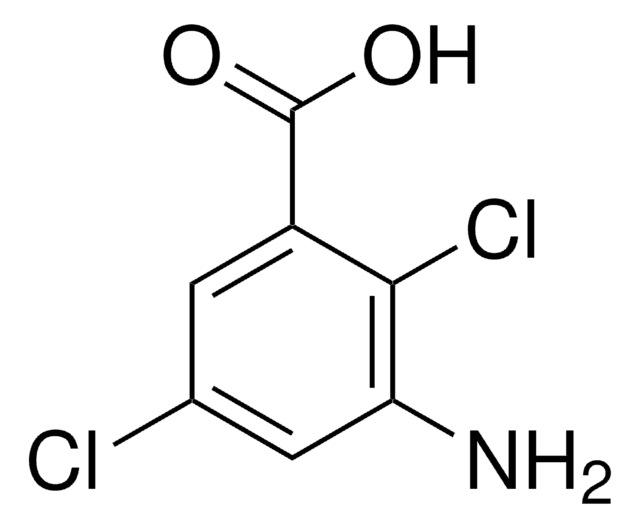
Linear Formula:
H2NC6H3(Cl)CO2H
CAS Number:
Molecular Weight:
171.58
Beilstein:
2803668
EC Number:
MDL number:
UNSPSC Code:
12352106
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
reaction suitability
reaction type: solution phase peptide synthesis
mp
211 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
Nc1ccc(C(O)=O)c(Cl)c1
InChI
1S/C7H6ClNO2/c8-6-3-4(9)1-2-5(6)7(10)11/h1-3H,9H2,(H,10,11)
InChI key
MBDUKNCPOPMRJQ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E H Philipson et al.
American journal of obstetrics and gynecology, 146(1), 16-22 (1983-05-01)
Most of the reports of fetal bradycardia and acidosis following paracervical block anesthesia have involved the use of amide-linked anesthetics, such as lidocaine and mepivacaine. The purposes of this study were (1) to determine placental transfer of an ester-linked local
G Menon et al.
Journal of pharmaceutical sciences, 73(2), 251-253 (1984-02-01)
A high-performance liquid chromatographic method has been developed for the simultaneous determination of chloroprocaine hydrochloride and its hydrolytic degradation product, 4-amino-2-chlorobenzoic acid. Separation is achieved using a mu-Bondapak C18 column and the eluant, water-acetonitrile-methanol-glacial acetic acid (74:20:5:1) containing 0.05-0.08% (w/v)
F Brun et al.
Journal of pharmaceutical and biomedical analysis, 14(8-10), 1251-1259 (1996-06-01)
The separation of HPLC of basic drugs on silica-based reversed phases remains a major problem because of the interaction between the residual silanol groups of the silica and the amino function of the drug. This paper describes the validation of
P K Janicki et al.
Journal of chromatography. B, Biomedical applications, 675(2), 336-341 (1996-01-26)
A sensitive and specific high-performance liquid chromatographic method for determination of the 2-chloroprocaine, local anesthetic of ester type, and its major metabolite 2-chloroaminobenzoic acid, has been developed and validated. A single-step extraction procedure is employed followed by high-performance liquid chromatographic
K Krohg et al.
Anesthesiology, 54(4), 329-332 (1981-04-01)
The purpose of this study was to identify the metabolic pathway of 2-chloro-4-aminobenzoic acid (CABA), a primary metabolite of chloroprocaine. Urine was collected from 4 healthy, pregnant women following the epidural administration of 600 mg chloroprocaine. The urinary metabolites were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service