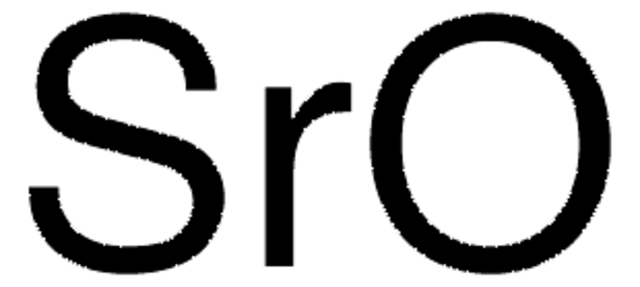204455
Strontium carbonate
99.995% trace metals basis
Synonym(s):
Strontianite
About This Item
Recommended Products
Assay
99.995% trace metals basis
form
powder and chunks
impurities
≤55.0 ppm Trace Metal Analysis
mp
1494 °C (lit.)
solubility
dilute aqueous acid: slightly soluble(lit.)
density
3.7 g/mL at 25 °C (lit.)
SMILES string
[Sr++].[O-]C([O-])=O
InChI
1S/CH2O3.Sr/c2-1(3)4;/h(H2,2,3,4);/q;+2/p-2
InChI key
LEDMRZGFZIAGGB-UHFFFAOYSA-L
Looking for similar products? Visit Product Comparison Guide
Application
- Strontium carbonate: Extensively used in the production of glazes in ceramics, where it acts as a flux and introduces strontium into the glaze for improved finish and durability. It is also crucial in the manufacture of ferrite magnets, providing the necessary strontium content to enhance magnetic properties. Additionally, its role in pyrotechnics is to contribute to the vibrant red hues seen in fireworks displays (Sigma-Aldrich, CAS 1633-05-2).
Storage Class Code
13 - Non Combustible Solids
WGK
nwg
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
The prevailing strategies for heat and electric-power production that rely on fossil and fission fuels are having a negative impact on the environment and on our living conditions.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service