All Photos(1)
About This Item
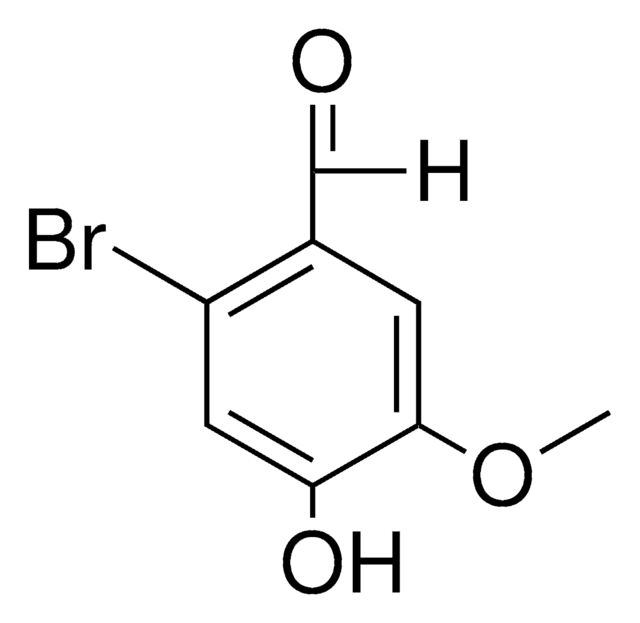
Linear Formula:
BrC6H2-4-(OH)-3-(OCH3)CHO
CAS Number:
Molecular Weight:
231.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
164-166 °C (lit.)
functional group
aldehyde
bromo
SMILES string
COc1cc(C=O)cc(Br)c1O
InChI
1S/C8H7BrO3/c1-12-7-3-5(4-10)2-6(9)8(7)11/h2-4,11H,1H3
InChI key
KLSHZDPXXKAHIJ-UHFFFAOYSA-N
Application
5-Bromovanillin was used to enrich the metabolically stable anaerobic cultures to study dechlorination of chlorocatechols. It was also used to prepare 2, 5-dihydroxy-4-methoxy-6-bromobenzaldehyde and 5-bromovanillate.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
P J Kersten et al.
Journal of bacteriology, 162(2), 693-697 (1985-05-01)
Four strains of gram-negative bacteria capable of growing at the expense of 5-chlorovanillate were isolated from soil, and the metabolism of one strain was studied in particular detail. In the presence of alpha, alpha'-bipyridyl, a suspension of 5-chlorovanillate-grown cells accumulated
G Martin et al.
European journal of biochemistry, 261(2), 533-539 (1999-04-24)
The Burkholderia cepacia AC1100 strain, known to degrade the herbicide, 2,4,5-Trichlorophenoxyacetic acid (2,4,5-T), is able to metabolize 4-hydroxyarylaldehyde, not only into the corresponding acid, but also into a new hydroquinone, 2,5-dihydroxyarylaldehyde. When incubated with resting AC1100 cells or cell-free extracts
Roland Tolulope Loto
Scientific reports, 7(1), 17555-17555 (2017-12-16)
The synergistic properties of the combined admixture of benzenecarbonitrile and 5-bromovanillin (BNV) on the corrosion resistance of 1018 carbon steel in 1 M HCl was analysed with potentiodynamic polarization technique, weight loss method, micro-analytical studies and ATF-FTIR spectroscopy. Results obtained show
A S Allard et al.
Applied and environmental microbiology, 57(1), 77-84 (1991-01-01)
Metabolically stable anaerobic cultures obtained by enrichment with 5-bromovanillin, 5-chlorovanillin, catechin, and phloroglucinol were used to study dechlorination of chlorocatechols. A high degree of specificity in dechlorination was observed, and some chlorocatechols were appreciably more resistant to dechlorination than others:
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service