126535
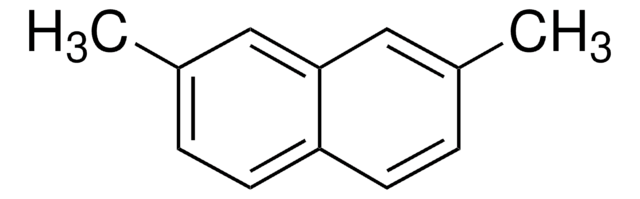
2,6-Dimethylnaphthalene
99%
Synonym(s):
2,6-Dimethylnaphthalene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H6(CH3)2
CAS Number:
Molecular Weight:
156.22
Beilstein:
1903544
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
bp
262 °C (lit.)
mp
106-110 °C (lit.)
SMILES string
Cc1ccc2cc(C)ccc2c1
InChI
1S/C12H12/c1-9-3-5-12-8-10(2)4-6-11(12)7-9/h3-8H,1-2H3
InChI key
YGYNBBAUIYTWBF-UHFFFAOYSA-N
Gene Information
human ... CYP1A2(1544)
Looking for similar products? Visit Product Comparison Guide
General description
2,6-dimethylnaphthalene is a polycyclic aromatic hydrocarbon available in the water bodies and can be determined by gas chromatography with flame-ionization.
Application
2,6-Dimethylnaphthalene hs been used as a substrate in intramolecular isotope effect experiments to compare substrate dynamics in CYP2E1 and CYP2A6.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A Rahman et al.
Canadian journal of physiology and pharmacology, 64(9), 1214-1218 (1986-09-01)
The mechanisms governing absorption of polynuclear aromatic hydrocarbons (PAHs) are important since these carcinogenic compounds occur as solutes in dietary lipids. These highly lipophilic compounds are well absorbed in the intestine. Bile salt micellar solubilization probably facilitates their transport across
J V Schnell et al.
Xenobiotica; the fate of foreign compounds in biological systems, 10(3), 229-234 (1980-03-01)
1. Benzo[a]pyrene, 2,6-dimethylnaphthalene, and naphthalene were used as substrates for a coho salmon (Oncorhynchus kisutch) liver microsomal preparation. 2. The apparent Michaelis constants (Km) were as follows: benzo[a]pyrene, 2.1 microM; 2,6-dimethylnaphthalene, 15.3 microM; and naphthalene, 300 microM. 3. The results
M M Krahn et al.
Journal of chromatography, 236(2), 441-452 (1982-02-19)
An automated extractor-concentrator was used to extract metabolites of naphthalene, 2,6-dimethylnaphthalene, and benzo[a]pyrene from serum, bile and liver homogenate of rainbow trout (Salmo gairdneri). The extracts were analyzed by reversed-phase high-performance liquid chromatography (HPLC) with fluorescence detection. Recoveries of naphthalene
N Miyachi et al.
Applied and environmental microbiology, 59(5), 1504-1506 (1993-05-01)
Three bacterial strains, identified as Alcaligenes sp. strain D-59 and Pseudomonas sp. strains D-87 and D-186, capable of growing on 2,6-dimethylnaphthalene (2,6-DMN) as the sole source of carbon and energy were isolated from soil samples. 2,6-Naphthalene dicarboxylic acid was formed
Dietary accumulation of dimethylnaphthalene by the grass shrimp Palaemonetes pugio under stable and fluctuating temperatures.
T M Dillon
Bulletin of environmental contamination and toxicology, 28(2), 149-153 (1982-02-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service