SML0011
VAHA
≥98% (HPLC)
Synonym(s):
VAHA, Valproyl hydroxamic acid, VPA-HA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H17NO2
CAS Number:
Molecular Weight:
159.23
MDL number:
UNSPSC Code:
12352200
PubChem Substance ID:
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
solubility
DMSO: ≥25 mg/mL
storage temp.
2-8°C
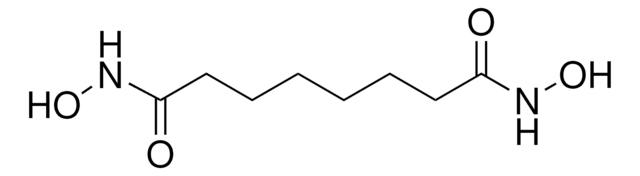
SMILES string
CCCC(CCC)C(=O)NO
InChI
1S/C8H17NO2/c1-3-5-7(6-4-2)8(10)9-11/h7,11H,3-6H2,1-2H3,(H,9,10)
InChI key
ROJGIRXXBBBMPL-UHFFFAOYSA-N
Application
VAHA may be used in cell signaling studies.
Biochem/physiol Actions
Hydroxamic acid derivatives of valproic acid exhibit anticonvulsant activity with no teratogenic activity in mouse neural tube defect model. It is effective in the treatment of bipolar disorders.
VAHA (Valproyl hydroxamic acid) is an HDAC inhibitor with less activity than valproic acid against Class I enzymes but much greater Class II activity
Features and Benefits
This compound is a featured product for Gene Regulation research. Click here to discover more featured Gene Regulation products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
B J Eickholt et al.
Molecular pharmacology, 67(5), 1426-1433 (2005-02-03)
Inositol-1,4,5-trisphosphate (InsP3) depletion has been implicated in the therapeutic action of bipolar disorder drugs, including valproic acid (VPA). It is not currently known whether the effect of VPA on InsP3 depletion is related to the deleterious effects of teratogenicity or
R Libert et al.
Biomedical & environmental mass spectrometry, 13(11), 599-603 (1986-11-01)
A previously unreported substance, detected by gas chromatography after oximation of urine from patients receiving valproic acid, was isolated and analysed by mass spectrometry and nuclear magnetic resonance spectroscopy. It was identified as the hydroxamate of valproic acid.
Ute Gravemann et al.
Neurotoxicology and teratology, 30(5), 390-394 (2008-05-06)
Fluorinated and non-fluorinated valproic acid (VPA) analogues with hydroxamic acid moieties were tested for their teratogenic, anticonvulsant and neurotoxic potencies in mice. Compounds were synthesized from their corresponding acids. The induction of neural tube defects (exencephaly) of the resulting hydroxamates
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service