S2076
α-2,6-Sialyltransferase from Photobacterium damsela
recombinant, expressed in E. coli BL21, ≥5 units/mg protein
Synonym(s):
β-Galactoside α-2,6-sialyltransferase, CMP-N-Acetylneuraminate:β-D-galactosyl-1,4-N-acetyl-β-D-glucosamine α-2,6-N-acetylneuraminyltransferase
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
recombinant
expressed in E. coli BL21
Quality Level
form
lyophilized powder
specific activity
≥5 units/mg protein
mol wt
56.8 kDa
shipped in
dry ice
storage temp.
−20°C
General description
Human ST6Gal-I (β-galactoside α-2,6-sialyltransferase 1) is a member of the CAZy family GT29.
Application
α-2,6-Sialyltransferase from Photobacterium damsela has been used in resialylation and restoration of sialic acids (SAs) in HRT-18G cells.
Highly active α2-6 sialyltransferase has been used to prepare high levels of disialylated fragment crystals.
Biochem/physiol Actions
The terminal step of complex N-glycan biosynthesis is catalysed by α-2,6-sialyltransferase (STs). Bacterial α(2,6)-STs possesses broader acceptor substrate specificity when compared to eukaryotic α(2,6)-STs.
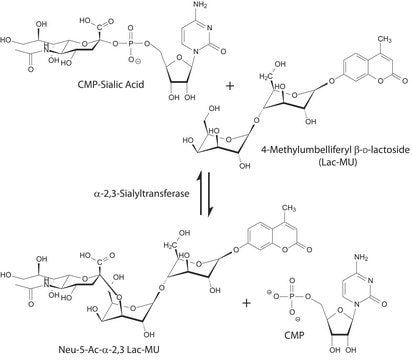
Sialyltransferase transfers Neu5Ac from CMP-Neu5Ac to the galactosyl terminus of acceptor molecules including glycoproteins, glycolipids, and oligosaccharides.
Unit Definition
One unit will catalyze the formation of 1 μmol Neu-5-Ac-α-2,6-LacMU from CMP-Neu-5-Ac and Lac-β−OMU per minute at 37 °C at pH 8.0.
Physical form
Supplied as a lyophilized powder containing Tris-HCl and NaCl.
Analysis Note
Enzymatic activity assays are performed in Tris-HCl buffer (100 mM, pH 8.0) containing CMP-Neu-5-Ac (1 mM) and Lac-β−OMU (1 mM) at 37 °C for 30 min and analyzed using HPLC with a fluorescence detector (excitation at 325 nm and emission at 372 nm).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Enhanced Bacterial alpha (2, 6)-Sialyltransferase Reaction through an Inhibition of Its Inherent Sialidase Activity by Dephosphorylation of Cytidine-5'-Monophosphate
Kang JY, et al.
PLoS ONE, 10(7), e0133739-e0133739 (2015)
Miyako Nakano et al.
Molecular & cellular proteomics : MCP, 10(11), M111-M111 (2011-08-24)
Resistance to tubulin-binding agents used in cancer is often multifactorial and can include changes in drug accumulation and modified expression of tubulin isotypes. Glycans on cell membrane proteins play important roles in many cellular processes such as recognition and apoptosis
Nageswari Yarravarapu et al.
Bioconjugate chemistry, 33(5), 781-787 (2022-04-20)
Glycan binding often mediates extracellular macromolecular recognition events. Accurate characterization of these binding interactions can be difficult because of dissociation and scrambling that occur during purification and analysis steps. Use of photocrosslinking methods has been pursued to covalently capture glycan-dependent
High-quality production of human alpha-2, 6-sialyltransferase in Pichia pastoris requires control over N-terminal truncations by host-inherent protease activities
Ribitsch D, et al.
Microbial cell factories, 13, 138-138 (2014)
Masayoshi Onitsuka et al.
Applied microbiology and biotechnology, 94(1), 69-80 (2011-12-30)
Improvement of glycosylation is one of the most important topics in the industrial production of therapeutic antibodies. We have focused on terminal sialylation with alpha-2,6 linkage, which is crucial for anti-inflammatory activity. In the present study, we have successfully cloned
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service