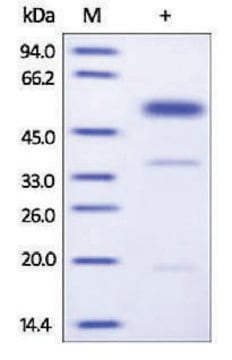
CS1070
Transglutaminase Assay Kit
sufficient for assays in two 96-well plates
Synonym(s):
TGases Assay Kit
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12161503
NACRES:
NA.28
Recommended Products
Quality Level
shipped in
dry ice
storage temp.
−20°C
Gene Information
human ... TGM1(7051) , TGM2(7052) , TGM3(7053) , TGM4(7047) , TGM5(9333) , TGM6(343641) , TGM7(116179)
General description
The Transglutaminase Assay Kit is designed for the detection of transglutaminase activity in biological samples. The kit can also be used for screening of transglutaminase inhibitors/activators.
Application
The assay is based on transglutaminase catalysis of a covalent bond formation between the free amine group of Poly L-Lysine, which is covalently attached to the plate surface, and the γ-carboxamide group of biotin-TVQQEL-OH substrate present in the assay buffer. This reaction immobilizes biotin to the plate surface. The amount of immobilized biotin is proportional to the amount of active transglutaminase in the sample. The amount of immobilized biotin is determined using streptavidin-peroxidase and TMB substrate.
Biochem/physiol Actions
Transglutaminases (TGases) are a widely distributed and unique group of calcium dependent enzymes that catalyze the post-translational modification of proteins by the formation of isopeptide bonds. This occurs either through protein cross-linking via formation of γ-glutamyl-ε-lysine bonds or through incorporation of primary amines at selected peptide-bound glutamine residues. The cross-linked products, often of high molecular mass, are highly resistant to mechanical challenge and proteolytic degradation, and their accumulation is found in a number of tissues and processes, where such properties are important including skin, hair, blood clotting, and wound healing. Deregulation of the enzyme activity contributes to a number of human diseases.
Other Notes
The detection limit is 0.003 mU/assay or 0.03mU/ml of Transglutaminase.
Kit Components Also Available Separately
Product No.
Description
SDS
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Resp. Sens. 1 - Skin Irrit. 2
Storage Class Code
8A - Combustible corrosive hazardous materials
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Bovine aortic endothelial cell transglutaminase. Enzyme characterization and regulation of activity.
G Korner et al.
The Biochemical journal, 262(2), 633-641 (1989-09-01)
Bovine aortic endothelial cells contain Ca2+-dependent tissue-type transglutaminase. Its activity in these cells was high, with apparent Km and Vmax. values with respect to putrescine of 0.203 mM and 18.5 nmol/min per mg of protein, and its activity was inhibited
Transglutaminases and their substrates.
Francesco Facchiano et al.
Progress in experimental tumor research, 38, 37-57 (2005-03-05)
Michael Cangkrama et al.
The Journal of investigative dermatology, 136(7), 1438-1448 (2016-03-16)
The skin barrier is critical for mammalian survival in the terrestrial environment, affording protection against fluid loss, microbes, toxins, and UV exposure. Many genes indispensable for barrier formation in the embryo have been identified, but loss of these genes in
Daniel Rittschof et al.
PloS one, 6(2), e16487-e16487 (2011-03-08)
Attachment strength of fouling organisms on silicone coatings is low. We hypothesized that low attachment strength on silicones is, in part, due to the interaction of surface available components with natural glues. Components could alter curing of glues through bulk
Mechanism of action of guinea pig liver transglutaminase. VII. Chemical and stereochemical aspects of substrate binding and catalysis.
S I Chung et al.
The Journal of biological chemistry, 245(23), 6424-6435 (1970-12-10)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service