936898
SPhos Pd G3 ChemBeads
Synonym(s):
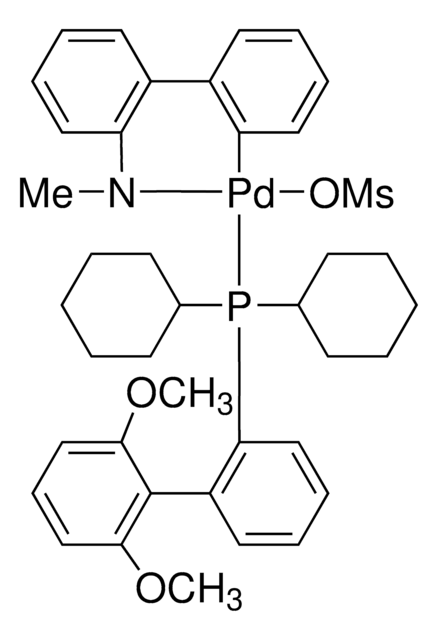
(2-Dicyclohexylphosphino-2′,6′-dimethoxybiphenyl) [2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate
About This Item
Recommended Products
form
solid
Quality Level
composition
, ~5 wt. % (loading of catalyst)
reaction suitability
reaction type: Buchwald-Hartwig Cross Coupling Reaction
reaction type: Cross Couplings
reaction type: Heck Reaction
reaction type: Hiyama Coupling
reaction type: Negishi Coupling
reaction type: Sonogashira Coupling
reaction type: Stille Coupling
reaction type: Suzuki-Miyaura Coupling
reagent type: catalyst
InChI
InChI: 1S/C26H35O2P.C12H10N.CH4O3S.Pd/c1-27-23-17-11-18-24(28-2)26(23)22-16-9-10-19-25(22)29(20-12-5-3-6-13-20)21-14-7-4-8-15-21;13-12-9-5-4-8-11(12)10-6-2-1-3-7-10;1-5(2,3)4;/h9-11,16-21H,3-8,12-15H2,1-2H3;1-6,8-9H,13H2;1H3,(H,2,3,4);/q;;;+1/p-1
InChI key
SCWODMZBSVVMRH-UHFFFAOYSA-M
General description
Application
For larger scale uses, product also available in powdered form (776246)
Other Notes
Versatile Methods to Dispense Sub-Milligram Quantities of Solids using Chemical Coated Beads for High-Throughput Experimentation
ChemBead Enabled High-Throughput Cross-Electrophile Coupling Reveals a New Complementary Ligand
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Sorry, we don't have COAs for this product available online at this time.
If you need assistance, please contact Customer Support.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service