51881
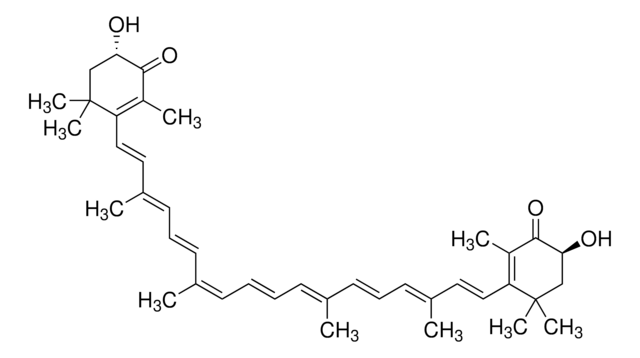
9-cis-Astaxanthin
analytical standard
Synonym(s):
(3S,3′S, 9-cis)-3,3′-Dihydroxy-β,β-carotene-4,4′-dione
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C40H52O4
CAS Number:
Molecular Weight:
596.84
UNSPSC Code:
85151701
NACRES:
NA.24
Recommended Products
grade
analytical standard
Quality Level
Assay
≥90.0% (HPLC)
shelf life
limited shelf life, expiry date on the label
technique(s)
HPLC: suitable
gas chromatography (GC): suitable
impurities
≤10% all-trans-Astaxanthin (HPLC)
application(s)
cleaning products
cosmetics
food and beverages
personal care
format
neat
storage temp.
−70°C
General description
9-cis-Astaxanthin is an isomer of astaxanthin (3,3 -dihydroxy-β,β-carotene-4,4 -dione), belonging to the xanthophyll family of oxygenated derivatives of carotenoids.
Application
- Detection and Analysis of Carbamazepine Metabolites: A study by Cui et al. used LC-MS/MS to detect carbamazepine and its metabolites, including 10,11-Dihydro-10-Hydroxycarbamazepine, in blood samples, indicating its utility as a pharmaceutical intermediate in biochemical assays and research on drug metabolism (Cui et al., 2023).
- Environmental Dynamics of Carbamazepine Metabolites: Research conducted by Malvar et al. focused on the dynamics of carbamazepine and its main metabolites in soil contamination studies, using methods like LC-MS/MS to trace pharmaceutical intermediates, providing insights into environmental biochemistry and pollution studies (Malvar et al., 2020).
- Serum Analysis for Drug Monitoring: Li et al. developed a solid-phase extraction method coupled with high-performance liquid chromatography to determine levels of carbamazepine and 10-Hydroxycarbamazepine in human serum, showcasing its application in clinical pharmacokinetics and therapeutic drug monitoring (Li et al., 2018).
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rapid baseline separation of enantiomers and a mesoform of all-trans-astaxanthin, 13-cis-astaxanthin, adonirubin, and adonixanthin in standards and commercial supplements.
Wang C, et al.
Journal of Chromatography A, 1194(2), 172-177 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service