8.56045
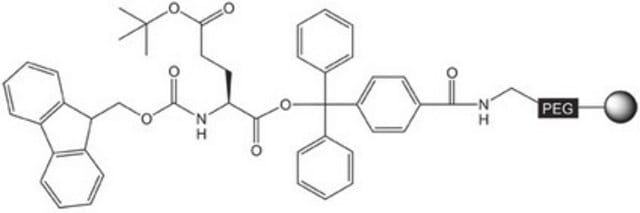
Fmoc-Gly-NovaSyn® TGT
for peptide synthesis, Novabiochem®
Synonym(s):
Fmoc-Glycine Resin
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
UNSPSC Code:
12352209
NACRES:
NA.22
Recommended Products
product name
Fmoc-Gly-NovaSyn® TGT, Novabiochem®
Quality Level
product line
NovaSyn® TG
Novabiochem®
form
beads
reaction suitability
reaction type: Fmoc solid-phase peptide synthesis
manufacturer/tradename
Novabiochem®
application(s)
peptide synthesis
functional group
Fmoc
storage temp.
15-25°C
General description
Pre-loaded resin for synthesis of peptide acids and protected peptide fragments containing a C-terminal glycine amino-acid residue by Fmoc SPPS.The base NovaSyn® TG is a composite of low cross-linked polystyrene and 3000-4000 M.W. polyethylene glycol. Peptide synthesis is carried out at the ends of the PEG chains that have been functionalized with the hyper-acid labile 4-carboxytrityl alcohol linker. This use of the bulky trityl-type linker helps prevent diketopiperazine formation that can occur during piperidine treatment of Fmoc-protected dipeptidyl resins.,,Treatment of the peptidyl resin with 20% TFE in DCM or 1% TFA in DCM cleaves the product from the resin without affecting the standard TFA-labile side-chain protecting groups. Standard TFA cleavage releases the fully deprotected peptide.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references:
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1993) Ann. Chem., 215.
[3] R. Steinauer, et al. in “Innovations & Perspectives in Solid Phase Synthesis”, R. Epton (Ed.), Mayflower Scientific Ltd., Birmingham, 1994, pp. 689.
[4] K. Barlos & D. Gatos in “Fmoc solid phase peptide synthesis: a practical approach”, W. C. Chan & P. D.White (Eds.), Oxford University Press, Oxford, 2000, pp. 218.
Associated Protocols and Technical Articles
Cleavage and Deprotection Protocols for Fmoc SPPS
Literature references:
[1] K. Barlos, et al. (1989) Tetrahedron Lett., 30, 3947.
[2] K. Barlos, et al. (1993) Ann. Chem., 215.
[3] R. Steinauer, et al. in “Innovations & Perspectives in Solid Phase Synthesis”, R. Epton (Ed.), Mayflower Scientific Ltd., Birmingham, 1994, pp. 689.
[4] K. Barlos & D. Gatos in “Fmoc solid phase peptide synthesis: a practical approach”, W. C. Chan & P. D.White (Eds.), Oxford University Press, Oxford, 2000, pp. 218.
Application
Fmoc-Gly-NovaSyn® TGT has been used to preparehydrazinopeptides as intermediates in the semi-synthesis of glycopeptides.
Linkage
Replaces: 04-12-2711
Analysis Note
Color (visual): white to yellow to beige
Appearance of substance (visual): beads
Loading (Photometric determination of the Fmoc-chromophore liberated upon treatment with DBU/DMF): 0.10 - 0.30 mmol/g
Swelling Volume (in DMF): lot specific result
Identity (of the substitution): passes test
The base resin is PEG-PS-copoymer (90µm), functionalised with 4-carboxy-tritylchloride.
Appearance of substance (visual): beads
Loading (Photometric determination of the Fmoc-chromophore liberated upon treatment with DBU/DMF): 0.10 - 0.30 mmol/g
Swelling Volume (in DMF): lot specific result
Identity (of the substitution): passes test
The base resin is PEG-PS-copoymer (90µm), functionalised with 4-carboxy-tritylchloride.
Legal Information
NOVASYN is a registered trademark of Merck KGaA, Darmstadt, Germany
Novabiochem is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
K. Barlos, et al.
Ann. Chem., 215-215 (1993)
Innovations & Perspectives in Solid Phase Synthesis
R. Steinauer, et al.
Solid Phase Synthesis, 2nd Int. Symposium, R. Epton, ed., 689-689 (1994)
Fmoc solid phase peptide synthesis: a practical approach
K. Barlos & D. Gatos
Solid Phase Peptide Synthesis: A Practical Approach, 218-218 (2000)
Investigation of acyl transfer auxiliary-assisted glycoconjugation for glycoprotein semi-synthesis
Nyandoro K, et al.
Organic & Biomolecular Chemistry, 20, 8506-8514 (2022)
Veresterung von partiell geschutzten peptid-fragmenten mit harzen. Einsatz von 2-chlortritylchlorid zur synthese von Leu15-gastrin I
K. Barlos, et al.
Tetrahedron Letters, 30, 3947-3947 (1989)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service