444249
MMP-2/MMP-9 Inhibitor II
The MMP-2/MMP-9 Inhibitor II, also referenced under CAS 193807-60-2, controls the biological activity of MMP-2/MMP-9. This small molecule/inhibitor is primarily used for Protease Inhibitors applications.
Synonym(s):
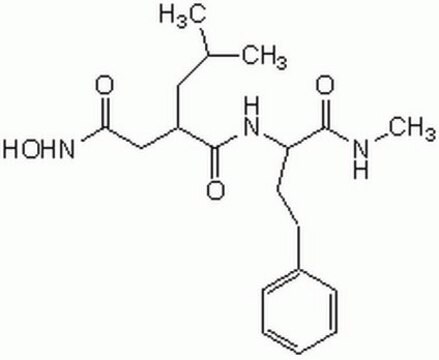
MMP-2/MMP-9 Inhibitor II, (2R)-[(4-Biphenylylsulfonyl)amino]-N-hydroxy-3-phenylpropionamide, BiPS, BiPS
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C21H20N2O4S
CAS Number:
Molecular Weight:
396.46
UNSPSC Code:
12352200
NACRES:
NA.77
Recommended Products
Quality Level
Assay
≥95% (HPLC)
form
solid
manufacturer/tradename
Calbiochem®
storage condition
OK to freeze
color
off-white
solubility
DMSO: 100 mg/mL
methanol: 25 mg/mL
shipped in
ambient
storage temp.
−20°C
General description
A potent inhibitor of MMP-2 (IC50 = 17 nM) and MMP-9 (IC50 = 30 nM). Also shown to potently induce and rapidly activate HIF. Reported to inhibit Lewis lung carcinoma-induced lung colonization in a murine model.
A potent inhibitor of type IV collagenases, MMP-2 (IC50 = 17 nM) and MMP-9 (IC50 = 30 nM). Also shown to potently induce and rapidly activate HIF. Has been shown in vivo to inhibit Lewis lung carcinoma-induced lung colonization in a murine model.
Biochem/physiol Actions
Cell permeable: no
Primary Target
MMP-2
MMP-2
Product does not compete with ATP.
Reversible: no
Target IC50: 17 nM, 30 nM against MMP-2, and MMP-9, respectively
Packaging
Packaged under inert gas
Warning
Toxicity: Standard Handling (A)
Reconstitution
Following reconstitution, aliquot and freeze (-20°C). Stock solutions are stable for up to 6 months at -20°C.
Other Notes
Lauzier, M.C., et al. 2008. Mol. Pharmacol.In press.
Mifune, M., et al. 2005. J. Biol. Chem.280, 26592.
Zhang, H., et al. 2004. Circ. Res.95, 989.
Tamura, Y., et al. 1998. J. Med. Chem.41, 640.
Mifune, M., et al. 2005. J. Biol. Chem.280, 26592.
Zhang, H., et al. 2004. Circ. Res.95, 989.
Tamura, Y., et al. 1998. J. Med. Chem.41, 640.
Legal Information
CALBIOCHEM is a registered trademark of Merck KGaA, Darmstadt, Germany
Storage Class Code
11 - Combustible Solids
WGK
WGK 1
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Shin Matsubara et al.
Bio-protocol, 10(7), e3577-e3577 (2021-03-05)
Ascidians are the closest living relatives of vertebrates ( Delsuc et al., 2006 ; Satoh et al., 2014 ) and are important for the evolutionary study of the ovarian follicle development including oocyte maturation and ovulation. However, neither the endogenous
E Kamanga-Sollo et al.
Domestic animal endocrinology, 58, 90-96 (2016-10-22)
In feedlot steers, estradiol-17β (E2) and combined E2 and trenbolone acetate (a testosterone analog) implants enhance rate and efficiency of muscle growth; and, consequently, these compounds are widely used as growth promoters in several countries. Treatment with E2 stimulates protein
Mehdi Benlarbi et al.
iScience, 25(11), 105316-105316 (2022-10-19)
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) spike glycoprotein (S) binds to angiotensin-converting enzyme 2 (ACE2) to mediate membrane fusion via two distinct pathways: 1) a surface, serine protease-dependent or 2) an endosomal, cysteine protease-dependent pathway. In this study, we found
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service