M89803
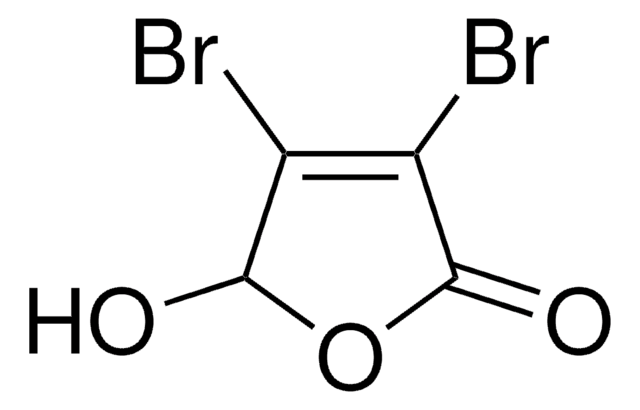
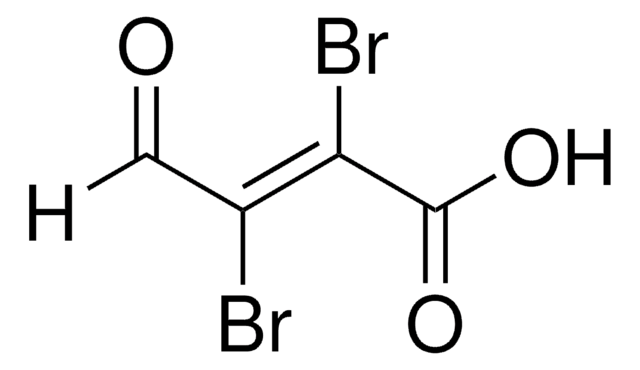
Mucochloric acid
99%
Synonym(s):
2,3-Dichloromalealdehydic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
OHCCCl=CClCOOH
CAS Number:
Molecular Weight:
168.96
Beilstein:
1705641
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
crystals
mp
125-128 °C (lit.)
SMILES string
OC(=O)\C(Cl)=C(/Cl)C=O
InChI
1S/C4H2Cl2O3/c5-2(1-7)3(6)4(8)9/h1H,(H,8,9)/b3-2+
InChI key
LUMLZKVIXLWTCI-NSCUHMNNSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Skin Corr. 1B - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1B - Non-combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point(F)
366.8 °F
Flash Point(C)
186 °C
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Oxidation and radical intermediates associated with the glutathione conjugation of mucochloric acid.
R T LaLonde et al.
Chemical research in toxicology, 7(4), 482-486 (1994-07-01)
The inactivation of the drinking water mutagen mucochloric acid (MCA) by reduced glutathione (GSH) was linked to the formation of an MCA-GSH conjugate, a nonmutagen in the Salmonella typhimurium (TA100) plate incorporation assay. Anaerobic formation of MCA-GSH is found now
L Kronberg et al.
Chemical research in toxicology, 5(6), 852-855 (1992-11-01)
Mucochloric acid, a genotoxic hydroxyfuranone found in chlorinated drinking water, was reacted with cytidine, adenosine, guanosine, and uridine. HPLC analyses with UV detection at 290 nm showed that one major product peak was formed in the reactions of cytidine, adenosine
Ji Zhang et al.
Organic letters, 5(4), 553-556 (2003-02-14)
[reaction: see text] The first direct reductive amination of mucochloric acid (1) has been accomplished. Reaction of 1 with various alkyl, aryl, and benzylamines, followed by reduction in the same pot, provides an efficient method of obtaining N-benzyl-3,4-dichloro-1,5-dihydro-pyrrol-2-one and N-aryl
I R Politzer et al.
Archives of environmental contamination and toxicology, 20(3), 371-374 (1991-04-01)
Cyclodextrins form inclusion complexes with a wide range of guest molecules which wholly, or in part, fit into their hydrophobic cavity. Since no covalent bonds are formed in this complexation, the guests can subsequently be eluted. The possibility of such
R T LaLonde et al.
Environmental and molecular mutagenesis, 22(3), 181-187 (1993-01-01)
A difference in biological response to enantiomers is not an uncommon observation and is, therefore, to be expected in various manifestations of genotoxicity. The bacterial mutagen mucochloric acid (2,3-dichloro-5-hydroxy-2(5H)-furanone) has one chiral center, at C-5, but this mutagen exists in
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service