All Photos(2)
About This Item
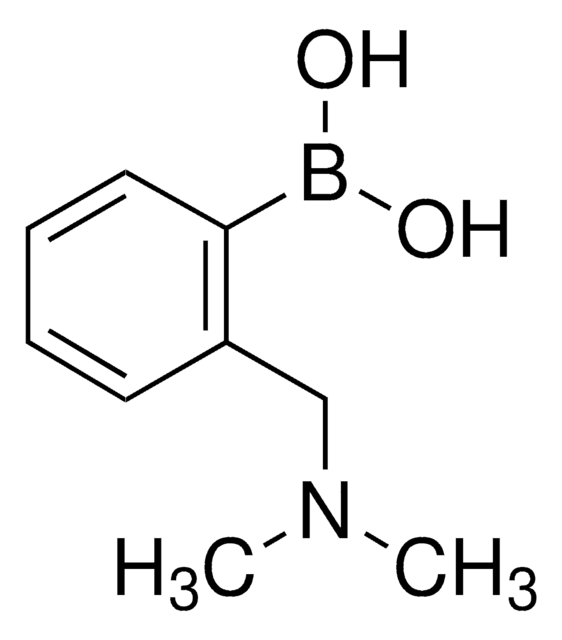
Empirical Formula (Hill Notation):
C8H12BNO2
CAS Number:
Molecular Weight:
165.00
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
≥95%
mp
178-190 °C
SMILES string
CN(C)c1cccc(c1)B(O)O
InChI
1S/C8H12BNO2/c1-10(2)8-5-3-4-7(6-8)9(11)12/h3-6,11-12H,1-2H3
InChI key
YZQQHZXHCXAJAV-UHFFFAOYSA-N
General description
May contain varying amounts of anhydride
Application
Reactant involved in synthesis of different protein effector including:
Reactant involved in synthesis of:
Reactant to undergo regioselective iodination and bromination
- Modulators of survival motor neuron protein
- Glucokinase activators
- Aryl ethers for use as Bacillus anthracis enoyl-ACP reductase inhibitors
Reactant involved in synthesis of:
- Thiourea-functionalized paracyclophanes
- Low-background fluorescent imaging agents for nitric oxide
Reactant to undergo regioselective iodination and bromination
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Gözde Murat Saltan et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 188, 372-381 (2017-08-02)
In an approach to develop efficient organic optoelectronic devices to be used in light-driven systems, a series of three thiophene linked benzimidazole conjugates were synthesized and characterized. The combination of two thiophene rings to a benzimidazole core decorated with different
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)