655228
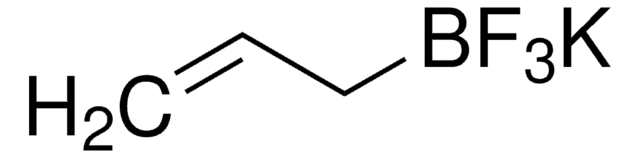
Potassium vinyltrifluoroborate
95%
Synonym(s):
Potassium (ethenyl)trifluoroborate
Sign Into View Organizational & Contract Pricing
All Photos(4)
About This Item
Linear Formula:
CH2=CHBF3K
CAS Number:
Molecular Weight:
133.95
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
SMILES string
[K+].F[B-](F)(F)C=C
InChI
1S/C2H3BF3.K/c1-2-3(4,5)6;/h2H,1H2;/q-1;+1
InChI key
ZCUMGICZWDOJEM-UHFFFAOYSA-N
General description
Potassium vinyltrifluoroborate is a versatile organometallic reagent used as a vinylating agent in the presence of palladium catalysts.
Potassium vinyltrifluoroborate is an air- and water-stable potassium organotrifluoroborate that can be utilized in coupling reactions under relatively mild conditions.
Potassium vinyltrifluoroborate is an air- and water-stable potassium organotrifluoroborate that can be utilized in coupling reactions under relatively mild conditions.
Application
Organotrifluoroborate involved in:
Organotrifluoroborates as versatile and stable boronic acid surrogates.
- Suzuki Miyaura cross-coupling reactions and polymerization reactions
- Synthesis of photonic crystals
- Synthesis of sensitizers for dye-sensitized solar cells
- Mannich / diastereoselective hydroamination reaction sequence
Organotrifluoroborates as versatile and stable boronic acid surrogates.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Potassium Ethenyl Trifluoroborate
Molander GA and Cooper DJ
eEROS (Encyclopedia of Reagents for Organic Synthesis) (2001)
The suzuki-heck polymerization as a tool for the straightforward obtainment of poly (fluorenylene-vinylene) sensitizers for dye-sensitized solar cells.
Grisorio R, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 49(4), 842-847 (2011)
New poly (phenylenevinylene)-methyl methacrylate-based photonic crystals.
Achelle S, et al.
Journal of Polymer Science Part A: Polymer Chemistry, 48(12), 2659-2665 (2010)
Suzuki- Miyaura cross-coupling reactions of potassium vinyltrifluoroborate with aryl and heteroaryl electrophiles.
Molander GA and Brown AR
The Journal of Organic Chemistry, 71(26), 9681-9686 (2006)
Xuejuan Ma et al.
Journal of hazardous materials, 355, 65-73 (2018-05-19)
The degradation of organophosphorous nerve agents is of primary concern due to the severe toxicity of these agents. Based on the active center of organophosphorus hydrolase (OPH), a bimetallic nuclear ligand, (5-vinyl-1,3-phenylene)bis(di(1H-imidazol-2-yl) methanol) (VPIM), was designed and synthesized, which contains
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II), complex with dichloromethane](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)
![[1,1′-Bis(diphenylphosphino)ferrocene]dichloropalladium(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)