530190
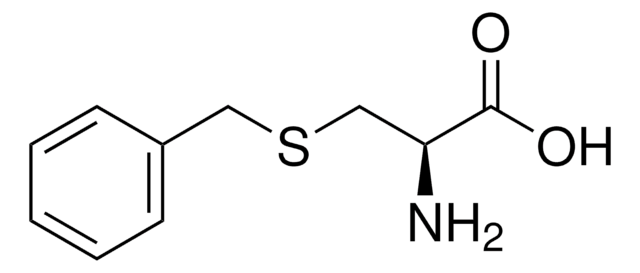
S-Phenyl-L-cysteine
97%
Synonym(s):
3-(Phenylthio)-L-Alanine, 4-Thia-L-homophenylalanine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
C6H5SCH2CH(NH2)CO2H
CAS Number:
Molecular Weight:
197.25
Beilstein:
2268203
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
optical activity
[α]20/D +10°, c = 1.5 in 1 M NaOH
reaction suitability
reaction type: solution phase peptide synthesis
mp
200 °C (dec.) (lit.)
application(s)
peptide synthesis
SMILES string
N[C@@H](CSc1ccccc1)C(O)=O
InChI
1S/C9H11NO2S/c10-8(9(11)12)6-13-7-4-2-1-3-5-7/h1-5,8H,6,10H2,(H,11,12)/t8-/m0/s1
InChI key
XYUBQWNJDIAEES-QMMMGPOBSA-N
Looking for similar products? Visit Product Comparison Guide
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
S M Rappaport et al.
Toxicology letters, 108(2-3), 117-126 (1999-10-08)
Cysteinyl adducts of hemoglobin (Hb) and albumin (Alb) formed via reactions with reactive species were measured in 48 subjects exposed to styrene (0.24-55.2 ppm) and to styrene-7,8-oxide (SO) (2.65-107 ppb) in a factory producing boats in the USA. Hb and
Martijn Rooseboom et al.
Chemical research in toxicology, 15(12), 1610-1618 (2002-12-17)
Tellurium compounds are effective antioxidants and chemoprotectors, even more active than their selenium and sulfur analogues. In addition to these properties, some selenium compounds, such as selenocysteine Se-conjugates, possess significant chemopreventive and antitumor activities, and selenol metabolites are considered as
W E Bechtold et al.
Archives of toxicology, 66(5), 303-309 (1992-01-01)
Benzene is metabolized to intermediates that bind to hemoglobin, forming adducts. These hemoglobin adducts may be usable as biomarkers of exposure. In this paper, we describe the development of a gas chromatography/mass spectroscopy assay for quantitating the binding of the
W E Bechtold et al.
Journal of toxicology and environmental health, 40(2-3), 377-386 (1993-10-01)
Three biomarkers for benzene exposure were developed. The first biomarker, muconic acid in urine, results from the ring opening of a benzene metabolite. A gas chromatography/mass spectroscopy (GC/MS) assay was developed to measure urinary muconic acid, and the analyte in
A A Melikian et al.
Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology, 1(4), 307-313 (1992-05-01)
The present study was aimed at the characterization of the major adducts formed by reaction of the metabolites of [14C]benzene with rat hemoglobin in vivo. Groups of 12-week-old male Fisher rats received i.p. injections of a single dose of 10
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service