All Photos(1)
About This Item
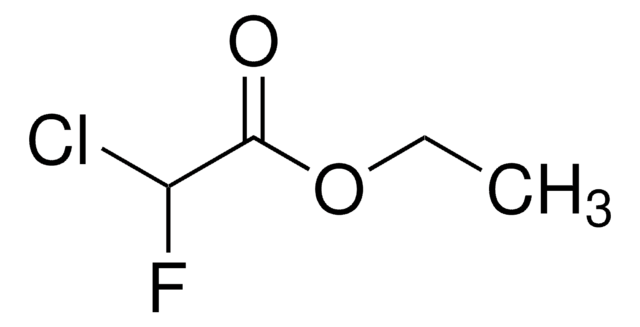
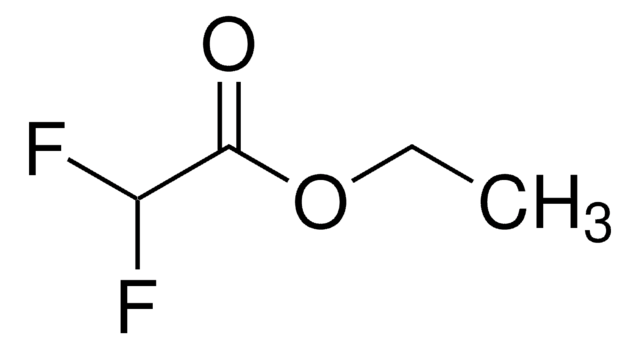
Linear Formula:
ClCF2CO2C2H5
CAS Number:
Molecular Weight:
158.53
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
refractive index
n20/D 1.358 (lit.)
bp
96-97.5 °C (lit.)
density
1.252 g/mL at 25 °C (lit.)
functional group
chloro
ester
fluoro
SMILES string
CCOC(=O)C(F)(F)Cl
InChI
1S/C4H5ClF2O2/c1-2-9-3(8)4(5,6)7/h2H2,1H3
InChI key
GVCAWQUJCHZRCB-UHFFFAOYSA-N
General description
Ethyl chlorodifluoroacetate (ECDFA) undergoes Reformatskii reaction with various aldehydes in DMF. It reacts with phenylacetylene to afford ethyl α,α−difluoro-4-phenyl-3-butenoates.
Application
Ethyl chlorodifluoroacetate (ECDFA) may be used as a starting reagent in the synthesis of 3-gem-difluoro-2-ethoxy allylic alcohols.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
64.4 °F - closed cup
Flash Point(C)
18 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
A theoretical investigation on the kinetics and reactivity of the gas-phase reactions of ethyl chlorodifluoroacetate with OH radical and Cl atom at 298 K.
Mishra BK, et al.
Structural Chemistry, 25(2), 463-470 (2014)
Fluorine-containing organozinc reagents. IV.: Reformatskii-type reactions of chlorodifluoroacet1c acid derivatives.
Lang RW and Schaub B.
Tetrahedron Letters, 29(24), 2943-2946 (1988)
Synthesis of 3-gem-difluoro-2-ethoxy allylic alcohols from ethyl chlorodifluoroacetate.
Begue J-P, et al.
Tetrahedron Letters, 35(3), 6097-6100 (1994)
Wadih Ghattas et al.
The Journal of organic chemistry, 71(22), 8618-8621 (2006-10-27)
An efficient preparation of pure ethyl Z- and E-alpha,alpha-difluoro-4-phenyl-3-butenoate 1a and 1b together with the corresponding acids 2a and 2b is described. The procedures involve stereocontrolled additions of *CF2CO2Et to phenylacetylene or beta-bromostyrene. Compound 1a is easily obtained by addition
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service