All Photos(1)
About This Item
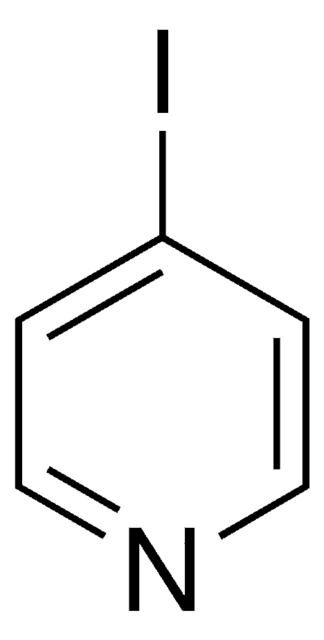
Linear Formula:
IC6H4NO2
CAS Number:
Molecular Weight:
249.01
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
bp
288-289 °C (lit.)
mp
49-51 °C (lit.)
functional group
iodo
nitro
SMILES string
[O-][N+](=O)c1ccccc1I
InChI
1S/C6H4INO2/c7-5-3-1-2-4-6(5)8(9)10/h1-4H
InChI key
JXMZUNPWVXQADG-UHFFFAOYSA-N
Application
1-Iodo-2-nitrobenzene was used in one step synthesis of 2-(2-Pyridyl)-3H-indol-3-one N-Oxide. 1-Iodo-2-nitrobenzene was used in the synthesis of 1-(2-Nitrophenyl)-1H-indole in the presence of PEG3400 (poly(ethylene glycol))–Cs2CO3–copper pre-catalyst under microwave activation.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Dermal - Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Dardan Hetemi et al.
Acta chimica Slovenica, dec(4), 818-824 (2018-01-11)
An efficient, versatile and non-destructive in situ method in reaction monitoring using vibrational spectroscopy is described. A Suzuki cross-coupling reaction was monitored in which the substrate 1-iodo-2-nitrobenzene reacted with the electrophile phenylboronic acid to form the product 2-nitrobiphenyl. To hasten
A one-step synthesis of 2-(2-Pyridyl)-3H-indol-3-one N-oxide: is it an efficient spin trap for hydroxyl radical?
G M Rosen et al.
The Journal of organic chemistry, 65(14), 4460-4463 (2000-07-13)
PEG3400-Cs2CO3: an efficient and recyclable microwave-enhanced catalytic system for ligand-free Ullmann arylation of indole and benzimidazole.
Colacino E, et al.
Tetrahedron, 66(21), 3730-3735 (2010)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service