推荐产品
等級
pharmaceutical primary standard
API 家族
paricalcitol
製造商/商標名
USP
應用
pharmaceutical (small molecule)
形式
neat
儲存溫度
−20°C
SMILES 字串
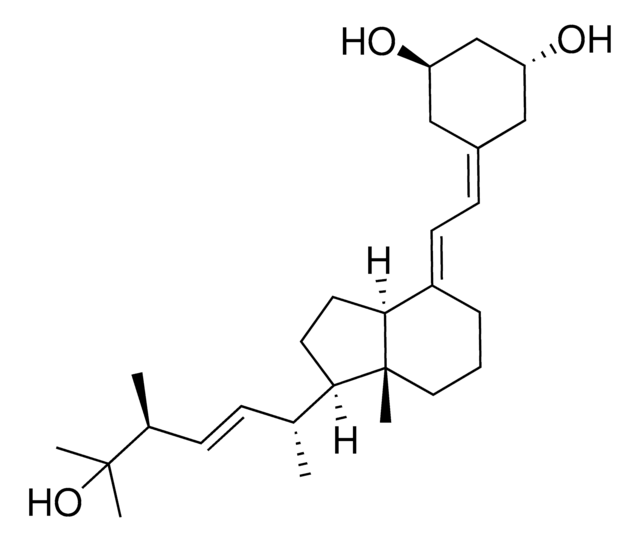
O[C@H]1C[C@@H](CC(=C\C=C2\[C@H]3[C@@]([C@H](CC3)[C@H](C)\C=C\[C@@H](C(O)(C)C)C)(CCC\2)C)C1)O
InChI
1S/C27H44O3/c1-18(8-9-19(2)26(3,4)30)24-12-13-25-21(7-6-14-27(24,25)5)11-10-20-15-22(28)17-23(29)16-20/h8-11,18-19,22-25,28-30H,6-7,12-17H2,1-5H3/b9-8+,21-11+/t18-,19+,22-,23-,24-,25+,27-/m1/s1
InChI 密鑰
BPKAHTKRCLCHEA-UBFJEZKGSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Paricalcitol USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
訊號詞
Danger
危險分類
Acute Tox. 2 Inhalation - Acute Tox. 3 Dermal - Acute Tox. 3 Oral - STOT RE 1 Oral
儲存類別代碼
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Ofer Havakuk et al.
The Israel Medical Association journal : IMAJ, 15(11), 693-697 (2014-02-12)
Vitamin D has been shown to induce beneficial effects on cardiovascular and renal morbidity by regulating inflammation and tissue fibrosis. To evaluate the effect of vitamin D analogues on cardiac function and fibrosis in an animal model of cardiorenal syndrome.
Vanessa Pérez et al.
European journal of pharmacology, 709(1-3), 72-79 (2013-04-10)
Secondary hyperparathyroidism is a common complication in patients with chronic kidney disease and frequently persists after kidney transplantation. Paricalcitol, a selective vitamin D receptor activator, is indicated in the management of this disorder and recent evidences have suggested that this
Jun Cheng et al.
Clinical journal of the American Society of Nephrology : CJASN, 7(3), 391-400 (2012-01-10)
Observational data indicate that newer vitamin D compounds such as paricalcitol can suppress serum intact parathyroid hormone (iPTH) and reduce proteinuria in patients with CKD. To systematically evaluate the efficacy and safety of paricalcitol for CKD, we conducted a meta-analysis
Angela Yee-Moon Wang et al.
Journal of the American Society of Nephrology : JASN, 25(1), 175-186 (2013-09-21)
Vitamin D seems to protect against cardiovascular disease, but the reported effects of vitamin D on patient outcomes in CKD are controversial. We conducted a prospective, double blind, randomized, placebo-controlled trial to determine whether oral activated vitamin D reduces left
Daniel W Coyne et al.
Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association, 28(9), 2260-2268 (2013-06-22)
Chronic kidney disease (CKD) is associated with elevations in serum phosphate, calcium-phosphorus product and bone-specific alkaline phosphatase (BAP), with attendant risks of cardiovascular and bone disorders. Active vitamin D can suppress parathyroid hormone (PTH), but may raise serum calcium and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门