推荐产品
等級
pharmaceutical primary standard
API 家族
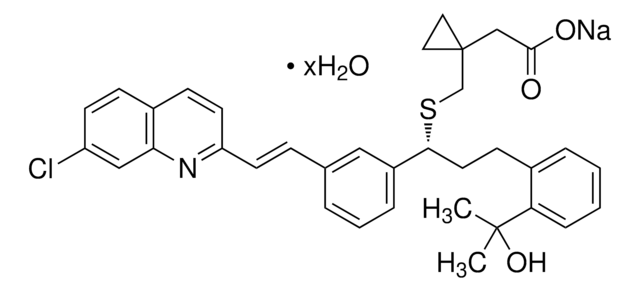
montelukast
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
InChI
1S/C35H36ClNO3S.Na/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29;/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39);/q;+1/p-1/b15-10+;/t32-;/m1./s1
InChI 密鑰
LBFBRXGCXUHRJY-HKHDRNBDSA-M
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Montelukast sodium USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia.
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
其他客户在看
Sheng-Hua Wu et al.
Pediatrics international : official journal of the Japan Pediatric Society, 56(3), 315-322 (2013-12-05)
Previous studies suggested that leukotrienes (LT) were involved in the pathogenesis of Henoch-Schönlein purpura (HSP). This study investigated the efficacy of an add-on therapy with montelukast in the treatment of HSP. In this four-center, double-blind, placebo-controlled, parallel paired comparative study
Yuki Ogawa et al.
European journal of pediatrics, 174(4), 509-518 (2014-09-25)
This study aimed to determine the population pharmacokinetics of doxapram in low-birth-weight (LBW) infants. A total of 92 serum concentration measurements that were obtained from 34 Japanese neonates were analyzed using nonlinear mixed-effect modeling (NONMEM). Estimates generated by NONMEM indicated
Om Prakash Ranjan et al.
Drug delivery, 21(7), 509-518 (2013-11-13)
The purpose of present study was to design, optimize and evaluate osmotically controlled pulsatile release capsule (PRC) of montelukast sodium (MKS) for the prevention of episodic attack of asthma in early morning and associated allergic rhinitis. Assembly of the capsular
Tarek A Ahmed et al.
AAPS PharmSciTech, 15(3), 772-780 (2014-03-22)
The objective of this study was to investigate the sustained release of a hydrophilic drug, montelukast (MK), from two biodegradable polymeric drug delivery systems, in situ implant (ISI) and in situ microparticles (ISM). N-Methyl pyrrolidone (NMP), dimethyl sulfoxide (DMSO), triacetin
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门