推荐产品
等級
pharmaceutical primary standard
API 家族
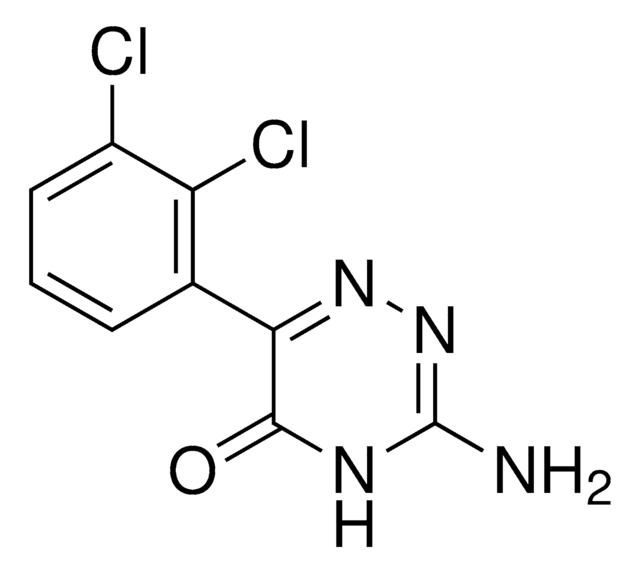
lamotrigine
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
SMILES 字串
Nc1nnc(c(N)n1)-c2cccc(Cl)c2Cl
InChI
1S/C9H7Cl2N5/c10-5-3-1-2-4(6(5)11)7-8(12)14-9(13)16-15-7/h1-3H,(H4,12,13,14,16)
InChI 密鑰
PYZRQGJRPPTADH-UHFFFAOYSA-N
基因資訊
human ... SCN10A(6336) , SCN11A(11280) , SCN1A(6323) , SCN2A(6326) , SCN3A(6328) , SCN4A(6329) , SCN5A(6331) , SCN7A(6332) , SCN8A(6334) , SCN9A(6335)
正在寻找类似产品? 访问 产品对比指南
生化/生理作用
抗惊厥药。
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Effect of Permeation enhancers on diffusion of lamotrigine drug through cellophane membrane.
Rao, Vinay, et al.
American Journal of Advanced Drug Delivery, 1.4, 606-610 (2013)
Allyson K Friedman et al.
Science (New York, N.Y.), 344(6181), 313-319 (2014-04-20)
Typical therapies try to reverse pathogenic mechanisms. Here, we describe treatment effects achieved by enhancing depression-causing mechanisms in ventral tegmental area (VTA) dopamine (DA) neurons. In a social defeat stress model of depression, depressed (susceptible) mice display hyperactivity of VTA
Philip J Wiffen et al.
The Cochrane database of systematic reviews, 12(12), CD006044-CD006044 (2013-12-04)
This is an update of the original Cochrane review entitled Lamotrigine for acute and chronic pain published in Issue 2, 2007, and updated in Issue 2, 2011. Some antiepileptic medicines have a place in the treatment of neuropathic pain (pain
Zhi-fei Wang et al.
Acta pharmacologica Sinica, 32(12), 1433-1445 (2011-11-08)
The mood stabilizers lithium, valproate and lamotrigine are traditionally used to treat bipolar disorder. However, accumulating evidence suggests that these drugs have broad neuroprotective properties and may therefore be promising therapeutic agents for the treatment of neurodegenerative diseases, including stroke.
Jennifer G Reid et al.
The Journal of clinical psychiatry, 74(7), 675-684 (2013-08-16)
Owing to the prevalence of medication side effects and treatment resistance, prescribers often consider off-label uses of US Food and Drug Administration (FDA)-approved agents for the treatment of persistent symptoms. The authors review the available literature on the FDA-approved and
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门