1098220
USP
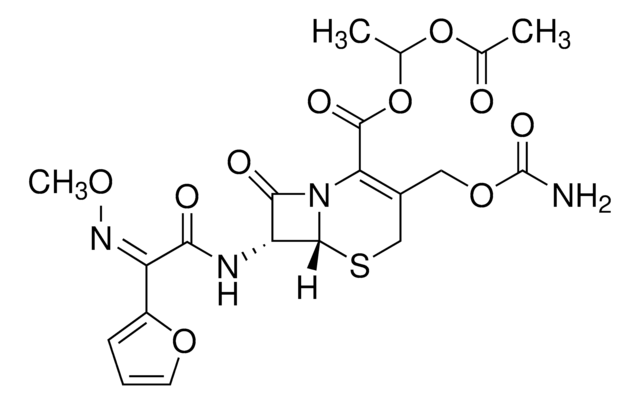
Cefuroxime Axetil
United States Pharmacopeia (USP) Reference Standard
别名:
[6R-[6α,7β(Z)]]-3-[[(Aminocarbonyl)oxy]methyl]-7-[[2-furanyl(methoxyimino)acetyl]amino]-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid 1-(acetyloxy)ethyl ester
登录查看公司和协议定价
所有图片(1)
About This Item
经验公式(希尔记法):
C20H22N4O10S
CAS号:
分子量:
510.47
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
cefuroxime
製造商/商標名
USP
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
O=C1[C@@H](NC(/C(C2=CC=CO2)=N\OC)=O)[C@]3([H])N1C(C(OC(C)OC(C)=O)=O)=C(COC(N)=O)CS3
InChI
1S/C20H22N4O10S/c1-9(25)33-10(2)34-19(28)15-11(7-32-20(21)29)8-35-18-14(17(27)24(15)18)22-16(26)13(23-30-3)12-5-4-6-31-12/h4-6,10,14,18H,7-8H2,1-3H3,(H2,21,29)(H,22,26)/b23-13-/t10?,14-,18-/m1/s1
InChI 密鑰
KEJCWVGMRLCZQQ-YJBYXUATSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Cefuroxime Axetil USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Cefuroxime Axetil for Oral Suspension
- Cefuroxime Axetil Tablets
分析報告
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Danger
危險聲明
危險分類
Resp. Sens. 1 - Skin Sens. 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Sruti Jammula et al.
Drug delivery, 22(1), 125-135 (2014-01-15)
Biphasic floating minitablets of cefuroxime axetil were prepared by melt granulation technique using two different grades of gelucire namely 50/13 and 43/01 to maintain constant plasma drug concentration. Loading dose of cefuroxime axetil was formulated as immediate release (IR) minitablets
P Gehanno et al.
The British journal of clinical practice, 49(1), 28-32 (1995-01-01)
Recent resurgence in serious streptococcal infections and rising failure rates with the standard 10-day course of therapy with penicillin V for group A beta-haemolytic streptococcus (GABHS)-associated tonsillopharyngitis (pharyngitis and/or tonsillitis) have heightened interest in alternative treatments for this infection. Reasons
L J Scott et al.
Drugs, 61(10), 1455-1500 (2001-09-18)
Cefuroxime axetil, a prodrug of the cephalosporin cefuroxime, has proven in vitro antibacterial activity against several gram-positive and gram-negative organisms, including those most frequently associated with various common community-acquired infections. In numerous randomised, controlled trials, 5 to 10 days' treatment
M A Marx et al.
Drug intelligence & clinical pharmacy, 22(9), 651-658 (1988-09-01)
Cefuroxime axetil is a orally active prodrug formulation of cefuroxime, which upon absorption undergoes immediate deesterification to free cefuroxime. Cefuroxime axetil offers an in vitro antibacterial spectrum against many gram-positive and some gram-negative organisms. Its beta-lactamase stability makes it useful
H J Manley et al.
Clinical nephrology, 49(4), 268-270 (1998-05-16)
Cefuroxime axetil has been associated with few reported adverse effects. We report a case of bilateral renal cortical necrosis in a female after receiving 7 doses over 4 treatment days. The patient presented with worsening symptoms consisting of arthralgias, pruritus
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门