所有图片(1)
About This Item
经验公式(希尔记法):
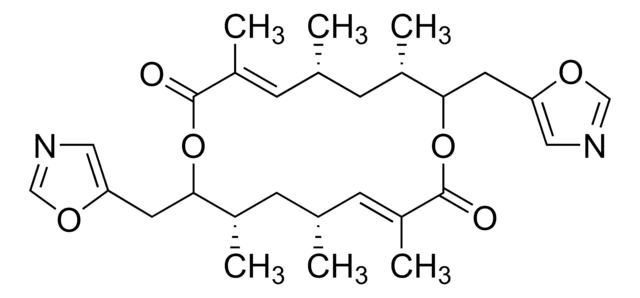
C18H28O4
CAS号:
分子量:
308.41
MDL號碼:
分類程式碼代碼:
12352205
PubChem物質ID:
NACRES:
NA.25
推荐产品
化驗
≥95% (LC/MS-ELSD)
形狀
solid
應用
metabolomics
vitamins, nutraceuticals, and natural products
儲存溫度
−20°C
InChI
1S/C18H28O4/c1-13-7-6-8-14(2)16(21-5)9-11-18(4,20)12-10-17(19)22-15(13)3/h8-13,15-16,20H,6-7H2,1-5H3/b11-9+,12-10+,14-8+/t13-,15+,16-,18+/m0/s1
InChI 密鑰
BYWWNDLILWPPJP-REXWONOSSA-N
一般說明
Natural product derived from fungal source.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Biosynthesis of albocycline: origin of the carbon skeleton.
A Taddei et al.
The Journal of antibiotics, 50(6), 526-528 (1997-06-01)
A new inhibitor of melanogenesis, albocycline K3, produced by Streptomyces sp. OH-3984.
S Takamatsu et al.
The Journal of antibiotics, 49(5), 485-486 (1996-05-01)
Albocycline- and carbomycin-type macrolides, inhibitors of human prolyl endopeptidases.
C Christner et al.
The Journal of antibiotics, 51(3), 368-371 (1998-05-20)
R C Thomas et al.
The Journal of antibiotics, 35(12), 1658-1664 (1982-12-01)
The structure and absolute configuration of the macrolide antibiotic albocycline (1a) has been determined by X-ray crystallographic analysis on the derived p-bromobenzoate (1b). The absolute configuration of albocycline is 4R, 7S, 12S, 13R.
K Harada et al.
The Journal of antibiotics, 37(10), 1187-1197 (1984-10-01)
As an approach to the search for new potentially useful macrolide antibiotics, we explored the minor components of albocycline (ALB) from the culture broth of Streptomyces bruneogriseus. Eight minor components were isolated and their structures were confirmed as 1 approximately
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门