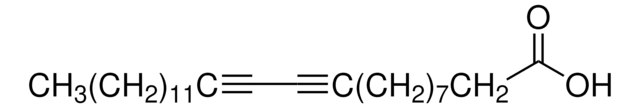所有图片(2)
About This Item
经验公式(希尔记法):
C18H32O2
CAS号:
分子量:
280.45
MDL號碼:
分類程式碼代碼:
12352106
PubChem物質ID:
NACRES:
NA.77
推荐产品
化驗
≥95% (GC)
形狀
powder
溶解度
chloroform: 10 mg/mL to clear, colorless to faintly yellow
SMILES 字串
C#CCCCCCCCCCCCCCCCC(O)=O
InChI
1S/C18H32O2/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-16-17-18(19)20/h1H,3-17H2,(H,19,20)
InChI 密鑰
DZIILFGADWDKMF-UHFFFAOYSA-N
應用
17-十八炔酸可用于脂质合成。
生化/生理作用
17-十八炔酸 (7-ODYA) 是细胞色素P450同工酶的不可逆抑制剂,参与长链脂肪酸代谢。
选择性和不可逆地抑制细胞色素 P450 环氧酶和 ω-水解酶的自杀底物抑制剂。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
其他客户在看
H Dong et al.
British journal of pharmacology, 120(4), 695-701 (1997-02-01)
1. The nature and cellular mechanisms that are responsible for endothelium-dependent relaxations resistant to indomethacin and NG-nitro-L-arginine methyl ester (L-NAME) were investigated in phenylephrine (PE) precontracted isolated carotid arteries from the rabbit. 2. In the presence of the cyclo-oxygenase inhibitor
Jacob S Yount et al.
Current protocols in chemical biology, 3(2), 65-79 (2011-05-01)
Protein fatty-acylation is the covalent addition of a lipid chain at specific amino acids. This modification changes the inherent hydrophobicity of a protein, often targeting it to cellular membrane compartments. Acylation may also regulate protein activity, stability, and protein-protein interactions.
Sphingosine-1-phosphate lyase deficient cells as a tool to study protein lipid interactions
Gerl M J, et al.
Testing, 11(4), e0153009-e0153009 (2016)
Effects of intrarenal infusion of 17-octadecynoic acid on renal antihypertensive mechanisms in anesthetized rabbits
Evans R G, et al.
American Journal of Hypertension, 11(7), 803-812 (1998)
M H Wang et al.
The Journal of pharmacology and experimental therapeutics, 284(3), 966-973 (1998-03-13)
We characterized the inhibitory activity of several acetylenic and olefinic compounds on cytochrome P450 (CYP)-derived arachidonic acid omega-hydroxylation and epoxidation using rat renal cortical microsomes and recombinant CYP proteins. Among the acetylenic compounds, 6-(2-propargyloxyphenyl)hexanoic acid (PPOH) and N-methylsulfonyl-6-(2-propargyloxyphenyl)hexanamide were found
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门

![三[(1-苄基-1H-1,2,3-三唑-4-基)甲基]胺 97%](/deepweb/assets/sigmaaldrich/product/structures/179/695/86a721c8-2a4c-4e4f-bc36-6276ce7a941f/640/86a721c8-2a4c-4e4f-bc36-6276ce7a941f.png)