所有图片(2)
About This Item
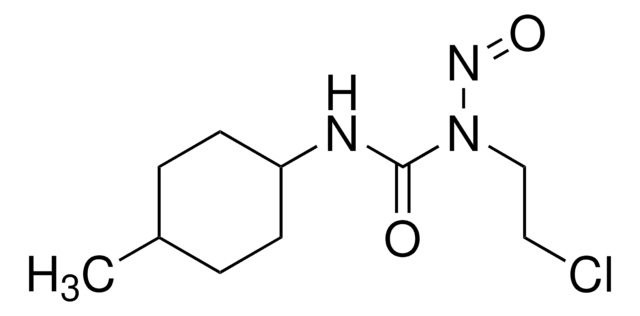
经验公式(希尔记法):
C9H16ClN3O2
CAS号:
分子量:
233.70
EC號碼:
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.25
推荐产品
生化/生理作用
Antineoplastic agent with cellular DNA effects. Lomustine induces p53 expression in A2870 cells.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 3 Oral - Carc. 1B
儲存類別代碼
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
水污染物質分類(WGK)
WGK 3
個人防護裝備
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
其他客户在看
Carsten Friedrich et al.
European journal of cancer (Oxford, England : 1990), 49(4), 893-903 (2012-11-28)
Medulloblastoma in adulthood is rare. Knowledge is limited, and the efficacy and toxicity of chemotherapy--especially in nonmetastatic disease--is still elusive. Seventy adults aged ≥21 years (median age: 28.5 years) with nonmetastatic medulloblastoma were followed as observational patients within the prospective
Enrico Franceschi et al.
Neuro-oncology, 14(12), 1503-1510 (2012-10-24)
The treatment of patients with recurrent glioblastoma remains a major oncologic problem, with median survival after progression of 7-9 months. To determine the maximum tolerated dose and dose-limiting toxicity (DLT), the combination of dasatinib and cyclonexyl-chloroethyl-nitrosourea (CCNU) was investigated in
Joann L Ater et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 30(21), 2641-2647 (2012-06-06)
PURPOSE Surgery is curative therapy for pediatric low-grade gliomas (LGGs) in areas of the brain amenable to complete resection. However, LGGs located in areas where complete resection is not possible can threaten both function and life. The purpose of this
Daisuke Hasegawa et al.
The Journal of veterinary medical science, 74(11), 1517-1521 (2012-07-13)
A 9 year-old, neutered, male French Bulldog showing cluster seizures was diagnosed with a glioma in the right piriform cortex by MRI. Hypofractionated radiation therapy (RT) was performed using a linear accelerator. Although the lesion had involuted significantly at 2
Tracy T Batchelor et al.
Journal of clinical oncology : official journal of the American Society of Clinical Oncology, 31(26), 3212-3218 (2013-08-14)
A randomized, phase III, placebo-controlled, partially blinded clinical trial (REGAL [Recent in in Glioblastoma Alone and With Lomustine]) was conducted to determine the efficacy of cediranib, an oral pan-vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor, either as monotherapy
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门