推荐产品
應用
HIV Protease Substrate 1 has been used:
- to study protein structure-function relationships and in the fluorescent assay
- to evaluate protease inhibitors and for in vitro detection of the HIV-1 protease activity
- and in protease kinetics to study its enzymatic degradation rates
生化/生理作用
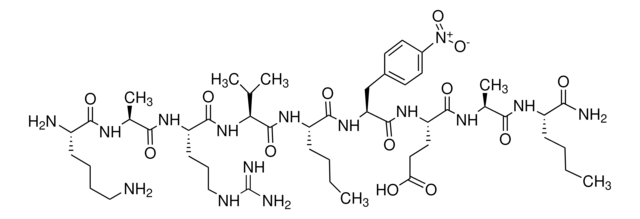
HIV Protease Substrate 1 is a synthetic peptide sequence that contains the cleavage site (Tyr-Pro) for the human immunodeficiency virus (HIV) Protease. It has two covalently modified amino acids for the detection of cleavage. One modification is the addition of the fluorophore 5-(2-aminoethylamino)-1-naphthalene sulfonate (EDANS) to the glutamic acid residue, while the other one includes the addition of the acceptor chromophore 4c-dimethylaminoazobenzene-4-carboxylate (DABCYL) to the lysine residue. The modified amino acids are on opposite sides of the cleavage site. Spatial orientation and overlap of the DABCYL absorbance with the EDANS emission permit resonance energy transfer between the two moieties. However, when the peptide is cleaved by the HIV protease, the DABCYL group is no longer proximal to the fluorophore.
Substrate for endopeptidases
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
Eyeshields, Gloves, type N95 (US)
Nancy Cheng et al.
Journal of laboratory automation, 19(3), 297-303 (2013-12-07)
Current antiretroviral treatments target multiple pathways important for human immunodeficiency virus (HIV) multiplication, including viral entry, synthesis and integration of the DNA provirus, and the processing of viral polyprotein precursors. However, HIV is becoming increasingly resistant to these "combination therapies."
Sagheer A Onaizi et al.
Langmuir : the ACS journal of surfaces and colloids, 23(11), 6336-6341 (2007-04-24)
We present the first study of the directed disassembly of a protein network at the air-water interface by the synergistic action of a surfactant and an enzyme. We seek to understand the fundamentals of protein network disassembly by using rubisco
Jason Segura et al.
PloS one, 18(2), e0281087-e0281087 (2023-02-14)
HIV infection remains incurable to date and there are no compounds targeted at the viral release. We show here HIV viral release is not spontaneous, rather requires caspases activation and shedding of its adhesion receptor, CD62L. Blocking the caspases activation
Rok Gaber et al.
Sensors (Basel, Switzerland), 13(12), 16330-16346 (2013-11-30)
To effectively fight against the human immunodeficiency virus infection/ acquired immunodeficiency syndrome (HIV/AIDS) epidemic, ongoing development of novel HIV protease inhibitors is required. Inexpensive high-throughput screening assays are needed to quickly scan large sets of chemicals for potential inhibitors. We
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门