所有图片(1)
About This Item
经验公式(希尔记法):
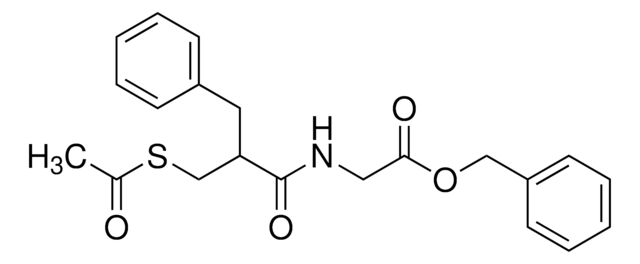
C21H23NO4S
CAS号:
分子量:
385.48
MDL號碼:
分類程式碼代碼:
41116107
PubChem物質ID:
NACRES:
NA.24
推荐产品
等級
pharmaceutical primary standard
API 家族
racecadotril
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
CC(=O)SCC(Cc1ccccc1)C(=O)NCC(=O)OCc2ccccc2
InChI
1S/C21H23NO4S/c1-16(23)27-15-19(12-17-8-4-2-5-9-17)21(25)22-13-20(24)26-14-18-10-6-3-7-11-18/h2-11,19H,12-15H2,1H3,(H,22,25)
InChI 密鑰
ODUOJXZPIYUATO-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Racecadotril for peak identification EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
生化/生理作用
Racecadotril is a neutral endopeptidase inhibitor, an antidiarrheal drug which acts as a peripherally acting enkephalinase inhibitor.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
訊號詞
Warning
危險聲明
危險分類
Acute Tox. 4 Oral - Aquatic Acute 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
J I Emparanza Knörr et al.
Anales de pediatria (Barcelona, Spain : 2003), 69(5), 432-438 (2009-01-09)
To estimate, through a systematic review of the literature, the efficacy of racecadotril in the treatment of acute diarrhoea. Randomised trials carried out in children comparing racecadotril with placebo in terms of diarrhoea recovery, stools output and adverse effects were
Ramón Tormo et al.
Acta paediatrica (Oslo, Norway : 1992), 97(8), 1008-1015 (2008-05-09)
In developing countries acute infectious diarrhoea remains one of the leading causes of death among young children, especially those under 1 year of age. In contrast, in industrialized nations the death rate is very low, although the disease is an
J M Lecomte
International journal of antimicrobial agents, 14(1), 81-87 (2000-03-16)
Since preclinical studies had indicated the potential efficacy and tolerability of racecadotril for the treatment of diarrhoea in man, a series of studies was carried out to assess the clinical effects of racecadotril. These studies were also designed to evaluate
J C Schwartz
International journal of antimicrobial agents, 14(1), 75-79 (2000-03-16)
Since enkephalins were discovered in 1975, it has become clear that they play an antisecretory role in the gastrointestinal tract. Hence a rational research programme was directed at the development of a drug that would preserve these neurotransmitter peptides in
A J Matheson et al.
Drugs, 59(4), 829-835 (2000-05-10)
Racecadotril is an oral enkephalinase inhibitor used in the treatment of acute diarrhoea. It prevents the degradation of endogenous opioids (enkephalins), thereby reducing hypersecretion of water and electrolytes into the intestinal lumen. In a randomised double-blind study in 6 adult
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门