推荐产品
等級
pharmaceutical primary standard
API 家族
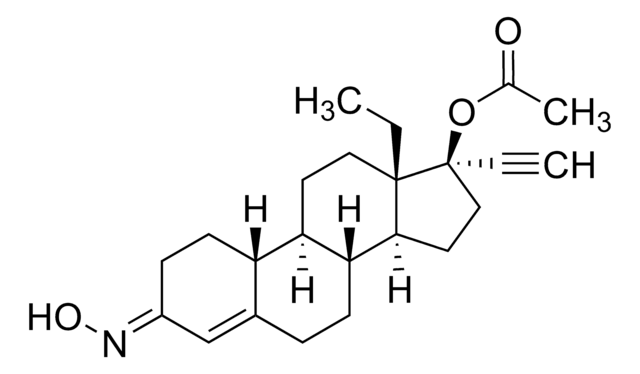
norgestimate
製造商/商標名
EDQM
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
CC[C@]12CC[C@H]3[C@@H](CCC4=C\C(CC[C@H]34)=N/O)[C@@H]1CC[C@@]2(OC(C)=O)C#C
InChI
1S/C23H31NO3/c1-4-22-12-10-19-18-9-7-17(24-26)14-16(18)6-8-20(19)21(22)11-13-23(22,5-2)27-15(3)25/h2,14,18-21,26H,4,6-13H2,1,3H3/b24-17+/t18-,19+,20+,21-,22-,23-/m0/s1
InChI 密鑰
KIQQMECNKUGGKA-NMYWJIRASA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
應用
Norgestimate EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
包裝
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
其他說明
Sales restrictions may apply.
相關產品
产品编号
说明
价格
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Eye Irrit. 2 - Repr. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
J L McGuire et al.
American journal of obstetrics and gynecology, 163(6 Pt 2), 2127-2131 (1990-12-01)
Biotransformation, pharmacologic, and pharmacokinetic studies of norgestimate and its metabolites indicate that 17-deacetyl norgestimate, along with the parent drug, contributes to the biologic response. The postulated metabolic pathway, which is based on the identification of urinary products had indicated that
M P Curran et al.
Drugs & aging, 18(11), 863-885 (2002-01-05)
The focus of this review is hormone replacement therapy (HRT) with continuous administration of micronised, oral 17beta-estradiol 1 mg/day (herein referred to as continuous estradiol) plus micronised, oral norgestimate 90 microg/day administered for 3 days then withdrawn for 3 days
S L Corson
American journal of obstetrics and gynecology, 170(5 Pt 2), 1556-1561 (1994-05-01)
Both monophasic and triphasic formulations of ethinyl estradiol plus norgestimate, a progestin with marked progesterone-receptor affinity and minimal androgen-receptor affinity, have been evaluated in numerous clinical studies designed to determine if norgestimate's receptor-binding profile provides enhanced safety without a reduction
Norgestimate: overview of a monophasic formulation.
F D Anderson et al.
International journal of fertility and menopausal studies, 38 Suppl 3, 126-132 (1993-01-01)
Monique P Curran et al.
Treatments in endocrinology, 1(2), 127-129 (2005-03-16)
The focus of this review is hormone replacement therapy (HRT) with continuous administration of micronized, oral 17beta-estradiol 1 mg/day (herein referred to as continuous estradiol) plus micronized, oral norgestimate 90 microg/day administered for 3 days then withdrawn for 3 days
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门