PHR1691
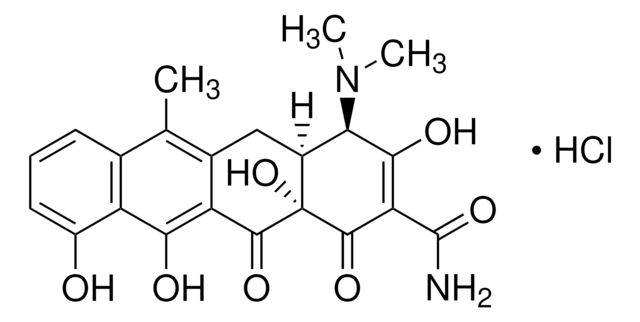
4-差向脱水四环素 盐酸盐
Pharmaceutical Secondary Standard; Certified Reference Material
别名:
(4R,4aS,12aS)-4-(二甲氨基)-1,4,4a,5,12,12a-六氢-3,10,11,12a-四羟基-6-甲基-1,12-二氧代-2-并四苯甲酰胺 单盐酸盐
About This Item
推荐产品
等級
certified reference material
pharmaceutical secondary standard
品質等級
agency
traceable to Ph. Eur. E0400000
traceable to USP 1236506
API 家族
chlortetracycline, tetracycline
CofA
current certificate can be downloaded
包裝
pkg of 500 mg
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
pharmaceutical (small molecule)
格式
neat
儲存溫度
2-8°C
SMILES 字串
Cl[H].[H][C@@]12Cc3c(C)c4cccc(O)c4c(O)c3C(=O)[C@]1(O)C(=O)C(C(N)=O)=C(O)[C@@H]2N(C)C
InChI
1S/C22H22N2O7.ClH/c1-8-9-5-4-6-12(25)13(9)17(26)14-10(8)7-11-16(24(2)3)18(27)15(21(23)30)20(29)22(11,31)19(14)28;/h4-6,11,16,25-27,31H,7H2,1-3H3,(H2,23,30);1H/t11-,16+,22-;/m0./s1
InChI 密鑰
XBSQEFHDCDFNJU-MOMXNFOMSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
應用
分析報告
其他說明
腳註
相關產品
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
其他客户在看
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门