推荐产品
等級
analytical standard
品質等級
產品線
PESTANAL®
儲存期限
limited shelf life, expiry date on the label
技術
HPLC: suitable
gas chromatography (GC): suitable
應用
agriculture
environmental
格式
neat
SMILES 字串
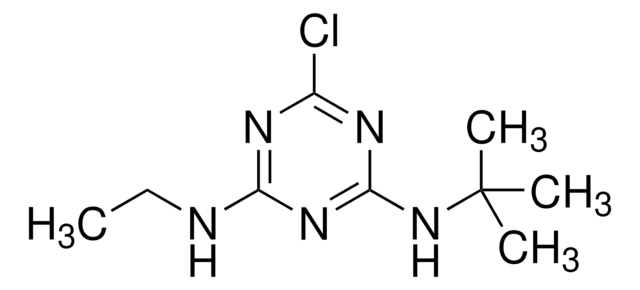
CCNc1nc(NC(C)(C)C)nc(SC)n1
InChI
1S/C10H19N5S/c1-6-11-7-12-8(15-10(2,3)4)14-9(13-7)16-5/h6H2,1-5H3,(H2,11,12,13,14,15)
InChI 密鑰
IROINLKCQGIITA-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
相关类别
一般說明
Terbutryn is primarily used to control a wide variety of annual grass and broadleaf weed species. This preemergence and postemergence s-triazine herbicide inhibits root development by interrupting mitosis.
應用
Refer to the product′s Certificate of Analysis for more information on a suitable instrument technique. Contact Technical Service for further support.
Terbutryn may be used as a reference standard for the determination of terbutryn in wastewater and soil by micellar liquid chromatography.
法律資訊
PESTANAL is a registered trademark of Merck KGaA, Darmstadt, Germany
訊號詞
Warning
危險聲明
危險分類
Aquatic Acute 1 - Aquatic Chronic 1
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Determination of diuron, terbuthylazine, and terbutryn in wastewater and soil by micellar liquid chromatography.
Pitarch-Andres S, et al.
Analytical and Bioanalytical Chemistry, 409(8), 2037-2049 (2017)
In vitro testing for genotoxicity of the herbicide terbutryn: cytogenetic and primary DNA damage.
Moretti M, et al.
Toxicology in vitro, 16(1), 81-88 (2002)
Effect of Pendimethalin, Trifluralin and Terbutryn on Lolium multiflorum growing with barley during pre-emergence stage.
Alshallash KS.
Annals of Agricultural Science, 59(2), 239-242 (2014)
Matthias Broser et al.
The Journal of biological chemistry, 286(18), 15964-15972 (2011-03-04)
Herbicides that target photosystem II (PSII) compete with the native electron acceptor plastoquinone for binding at the Q(B) site in the D1 subunit and thus block the electron transfer from Q(A) to Q(B). Here, we present the first crystal structure
Kristin Quednow et al.
Environmental science and pollution research international, 16(6), 630-640 (2009-05-23)
The present study focuses on the temporal concentration changes of four common organic pollutants in small freshwater streams of Hesse, Germany. The substances (tris(2-chloroethyl)phosphate (TCEP), the technical isomer mixture of 4-nonylphenol (NP), 2-(t-butylamino)-4-(ethylamino)-6-(methylthio)-s-triazine (terbutryn), and N,N-diethyl-m-toluamide (DEET)) are subject to
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门