推荐产品
品質等級
化驗
97%
折射率
n20/D 1.396 (lit.)
bp
106 °C (lit.)
密度
0.801 g/mL at 25 °C (lit.)
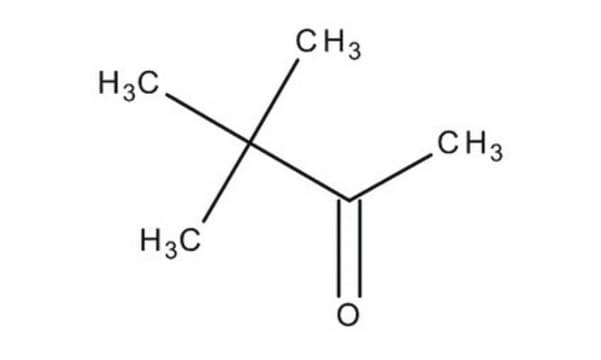
SMILES 字串
CC(=O)C(C)(C)C
InChI
1S/C6H12O/c1-5(7)6(2,3)4/h1-4H3
InChI 密鑰
PJGSXYOJTGTZAV-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
3,3-Dimethyl-2-butanone is an aliphatic ketone can undergo asymmetric reduction to the corresponding alcohol with diisopinocampheylchloroborane with high enantiomeric excess.
訊號詞
Danger
危險聲明
危險分類
Acute Tox. 4 Oral - Flam. Liq. 2
儲存類別代碼
3 - Flammable liquids
水污染物質分類(WGK)
WGK 2
閃點(°F)
41.0 °F - closed cup
閃點(°C)
5 °C - closed cup
個人防護裝備
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
其他客户在看
Y Chen et al.
The Journal of organic chemistry, 66(11), 3930-3939 (2001-05-26)
To investigate the effects of electron-donating and electron-withdrawing substituents upon the reaction of porphyrins with osmium tetraoxide, and the pinacol-pinacolone rearrangement of the resulting diols, a series of meso-substituted porphyrins were prepared by total synthesis. Porphyrins with electron-donating substitutents at
Kosuke Namba et al.
Organic letters, 14(5), 1222-1225 (2012-02-22)
An efficient method for the construction of dihydroquinoline derivatives possessing a quaternary carbon center is developed by an application of Hg(OTf)(2)-catalyzed vinylogous semi-pinacol-type rearrangement. The reaction was found to be specifically catalyzed by mercury salt and to proceed via a
Highly efficient asymmetric reduction of. alpha.-tertiary alkyl ketones with diisopinocampheylchloroborane.
Brown H C, et al.
The Journal of Organic Chemistry, 51(17), 3394-3396 (1986)
Direct conversion of arylamines to pinacol boronates: a metal-free borylation process.
Fanyang Mo et al.
Angewandte Chemie (International ed. in English), 49(10), 1846-1849 (2010-02-04)
Makoto Shimizu et al.
Organic letters, 4(23), 4097-4099 (2002-11-09)
The pinacol reaction of beta-halogenated alpha,beta-unsaturated aldehydes was promoted by titanium tetraiodide to give coupling products in good yields with high dl-selectivity. Subsequent reduction with H(2)/Pd-C gave saturated vic-diols in good yields. Heck coupling reaction enabled the displacement of halogens
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门