推荐产品
一般說明
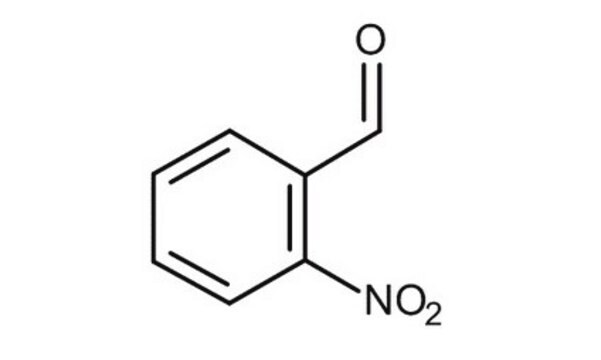
2-硝基苯甲醛中的2-硝基苄基具有光不稳定性,当暴露于紫外光时会发生裂解。
應用
2-硝基苯甲醛可与壳聚糖反应形成2-硝基苄基-壳聚糖,其可溶于三氟乙酸,也可电纺成纳米纤维基质。在光解时,硝基苄基被裂解,以获得纯的壳聚糖纳米纤维基质。相同的原理已被应用于明胶纳米纤维基质的制备。o-NBA与2-氨基苯并噻唑的缩合反应可形成 o-硝基亚苄基-2-氨基苯并噻唑,其是一种Schiff碱,可与金属离子反应形成络合物。
訊號詞
Warning
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 2
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
A smart methodology to fabricate electrospun chitosan nanofiber matrices for regenerative engineering applications.
Nada A A, et al.
Polymers For Advanced Technologies, 25(5), 507-515 (2014)
Gelatin nanofiber matrices derived from schiff base derivative for tissue engineering applications.
Jaiswal D, et al.
Journal of Biomedical Nanotechnology, 11(11), 2067-2080 (2015)
Two-photon photolysis of 2-nitrobenzaldehyde monitored by fluorescent-labeled nanocapsules.
Diaspro A, et al.
The Journal of Physical Chemistry B, 107(40), 11008-11012 (2003)
Fang-Bo Yu et al.
Journal of hazardous materials, 176(1-3), 20-26 (2009-07-07)
This study demonstrates the feasibility of using Pseudomonas putida ONBA-17 to bioaugment a sequencing batch reactor (SBR) treating o-nitrobenzaldehyde (ONBA) synthetic wastewater. To monitor its survival, the strain was chromosomally marked with gfp gene. After a transient adaptation, almost 100%
Annapaola Migani et al.
Chemical communications (Cambridge, England), 47(22), 6383-6385 (2011-05-10)
o-Nitrobenzaldehyde is photolabile because of an irreversible phototautomerization, whereas comparable aromatic compounds function as photoprotectors because the tautomerization is reversible. In this experimental and theoretical study we track down the cause of this difference to the electronic changes that occur
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门