推荐产品
化驗
97%
形狀
powder
mp
236-238 °C (dec.) (lit.)
SMILES 字串
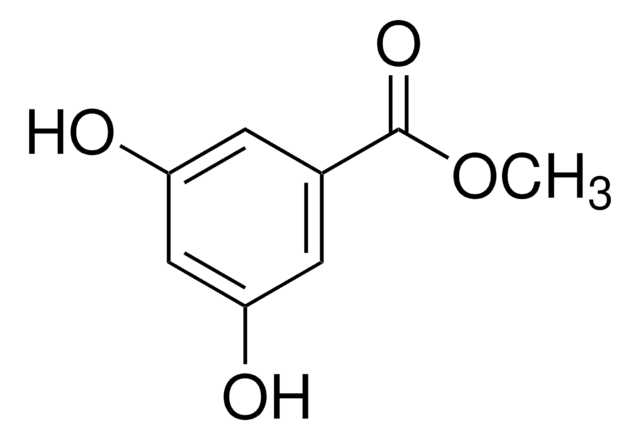
OC(=O)c1cc(O)cc(O)c1
InChI
1S/C7H6O4/c8-5-1-4(7(10)11)2-6(9)3-5/h1-3,8-9H,(H,10,11)
InChI 密鑰
UYEMGAFJOZZIFP-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 1
閃點(°F)
392.0 °F - closed cup
閃點(°C)
200 °C - closed cup
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
Sunil Varughese et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 12(6), 1597-1609 (2005-10-27)
A molecular recognition study of 3,5-dihydroxybenzoic acid (1) and its bromo derivative 4-bromo-3,5-dihydroxybenzoic acid (2) with the N-donor compounds 1,2-bis(4-pyridyl)ethene (bpyee), 1,2-bis(4-pyridyl)ethane (bpyea), and 4,4'-bipyridine (bpy) is reported. Thus, the syntheses and structural analysis of molecular adducts 1 a-1 c
Anja Koskela et al.
Clinical chemistry, 53(7), 1380-1383 (2007-05-15)
Whole-grain rye and wheat cereals contain high amounts of alkylresorcinols (ARs), phenolic lipids. ARs can be quantified in plasma. Two recently identified urinary AR metabolites, 3,5-dihydroxyphenylbenzoic acid (DHBA) and 3-(3,5-dihydroxyphenyl)-1-propanoic acid (DHPPA), may be useful as biomarkers of intake of
Yingdong Zhu et al.
The Journal of nutrition, 144(2), 114-122 (2013-11-22)
Biomarkers of dietary intake are prominent tools in nutritional research. The alkylresorcinol metabolites 3,5-dihydroxybenzoic acid (3,5-DHBA) and 3-(3,5-dihydroxyphenyl)propanoic acid (3,5-DHPPA) have been proposed as exposure biomarkers of whole-grain (WG) wheat and rye intake. However, the profile of alkylresorcinol metabolites is
Yasuyo Seshime et al.
Bioorganic & medicinal chemistry letters, 20(16), 4785-4788 (2010-07-16)
As a novel superfamily of type III polyketide synthases in microbes, four genes csyA, csyB, csyC, and csyD, were found in the genome of Aspergillus oryzae, an industrially important filamentous fungus. In order to analyze their functions, we carried out
Abigail E Wolfe et al.
Biochemistry, 46(19), 5741-5753 (2007-04-21)
Dihydroorotate dehydrogenases (DHODs) catalyze the oxidation of dihydroorotate to orotate in the only redox reaction in pyrimidine biosynthesis. The pyrimidine binding sites are very similar in all structurally characterized DHODs, suggesting that the prospects for identifying a class-specific inhibitor directed
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门