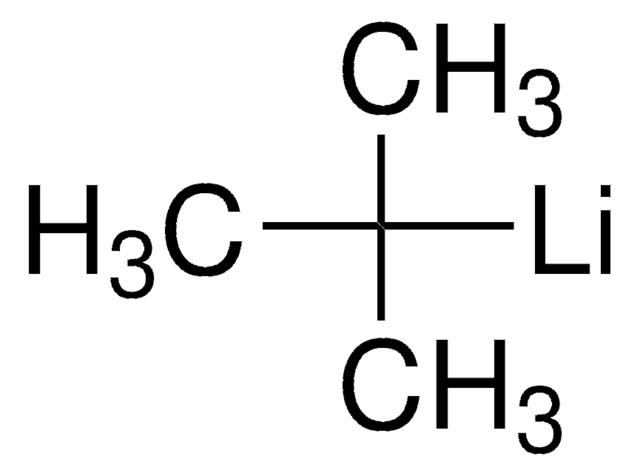
推荐产品
形狀
liquid
儲存溫度
2-8°C
應用
This benzyl boronate tag is a synthetic means for the subcellular targeting of cargo to the nucleus. Intracellular targeting can be important for understanding the localization of metabolites, proteins, or chemical probes or to increase therapeutic efficacy by concentrating a drug at its site of action and reducing off-target effects. Localization specifically to the nucleus is typically achieved using peptide localization signals and/or relies on passive diffusion. It was recently demonstrated, however, that nuclear targeting could be accomplished instead with a small-molecule motif benzyl boronic acid via synergistic active and passive transport processes. Tang, et al, presented examples using this tag to deliver proteins to the nucleus by the importin α/β pathway, including fluorescent proteins, ribonuclease A (RNase A), and chymotrypsin. The conjugation of this nucleus-targeting building block to other proteins or small molecules will facilitate nuclear localization in varied chemical biology experiments.
相關產品
产品编号
说明
价格
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Rui Tang et al.
Journal of the American Chemical Society, 139(25), 8547-8551 (2017-06-10)
Active intracellular transport is a central mechanism in cell biology, directed by a limited set of naturally occurring signaling peptides. Here, we report the first nonpeptide moiety that recruits intracellular transport machinery for nuclear targeting. Proteins synthetically modified with a
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)](/deepweb/assets/sigmaaldrich/product/structures/130/734/8846aa26-1858-458a-998d-8c306c13bf0f/640/8846aa26-1858-458a-998d-8c306c13bf0f.png)

![[1,1′-双(二苯基膦)二茂铁]二氯化钯(II)二氯甲烷络合物](/deepweb/assets/sigmaaldrich/product/structures/825/986/4317978b-1256-4c82-ab74-6a6a3ef948b1/640/4317978b-1256-4c82-ab74-6a6a3ef948b1.png)