所有图片(1)
About This Item
经验公式(希尔记法):
C9H13NO
CAS号:
分子量:
151.21
Beilstein:
2413029
MDL號碼:
分類程式碼代碼:
12352112
PubChem物質ID:
NACRES:
NA.22
推荐产品
等級
produced by BASF
品質等級
化驗
≥98.5% (GC)
99%
形狀
liquid
光學純度
enantiomeric excess: ≥98.5%
密度
1.024 g/mL at 20 °C (lit.)
官能基
amine
SMILES 字串
COc1ccc(cc1)[C@@H](C)N
InChI
1S/C9H13NO/c1-7(10)8-3-5-9(11-2)6-4-8/h3-7H,10H2,1-2H3/t7-/m1/s1
InChI 密鑰
JTDGKQNNPKXKII-SSDOTTSWSA-N
正在寻找类似产品? 访问 产品对比指南
應用
(R)-(+)-4-Methoxy-α-methylbenzylamine can be used as a reactant to prepare:
- Enantiopure stereoisomers of hemicryptophanes, which are used for the recognition of glucopyranosides.
- Bicyclic Geissman-Waiss lactone via intramolecular ring-closure reaction of the diastereomeric mixture of sulfonium salts.
- N-[(1R)-1-(4-Methoxyphenyl)ethyl]-N′-methylthiourea by reacting with methyl isothiocyanate.
法律資訊
ChiPros is a registered trademark of BASF SE
訊號詞
Danger
危險分類
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B - Skin Sens. 1A
儲存類別代碼
8A - Combustible corrosive hazardous materials
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
Improved hemicryptophane hosts for the stereoselective recognition of glucopyranosides
Schmitt A, et al.
Organic & Biomolecular Chemistry, 12(24), 4211-4217 (2014)
Synthesis of (+)-and (-)-Geissman-Waiss lactone from chiral sulfonium salts
Lopez-Gonzalez R, et al.
Tetrahedron Letters, 12(24), 151697-151697 (2020)
Yohan Park et al.
Archives of pharmacal research, 35(8), 1393-1401 (2012-09-04)
Thirty two thiourea derivatives were prepared and their agonistic activities on the retinoic acid receptor-related orphan receptor α (RORα) were evaluated. The replacement of the 3-allyl-2-imino-thiazolidin-4-one moiety of the lead compound CGP52608 (1) with various functional group substituted aromatic rings
商品
Chiral amines play an important role in stereoselective organic synthesis. They are used directly as resolving agents, building blocks, or chiral auxiliaries.
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门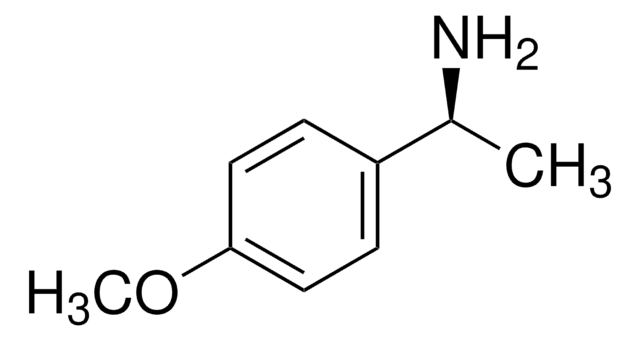

![Pentaerythritol tetrakis[2-(dodecylthiocarbonothioylthio)-2-methylpropionate] 97% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/234/301/a6e20d26-df1b-49c6-bdee-c98dd3488cc2/640/a6e20d26-df1b-49c6-bdee-c98dd3488cc2.png)






