推荐产品
化驗
99.0%
mp
168-175 °C (lit.)
SMILES 字串
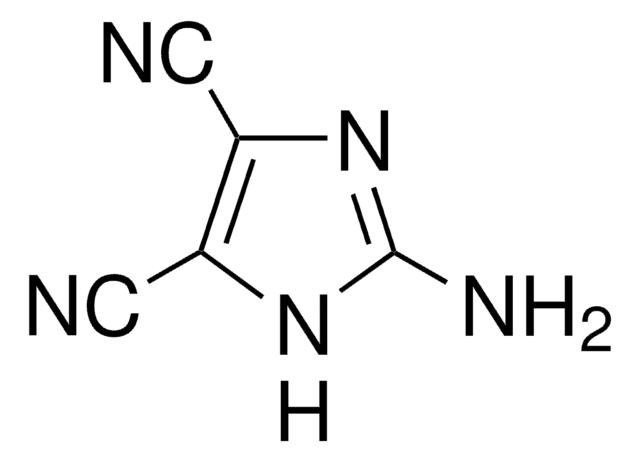
N#Cc1nc[nH]c1C#N
InChI
1S/C5H2N4/c6-1-4-5(2-7)9-3-8-4/h3H,(H,8,9)
InChI 密鑰
XGDRLCRGKUCBQL-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
一般說明
4,5-二氰基咪唑(DCI)可作为寡核苷酸合成中的活化剂。
應用
4,5-二氰基咪唑可用于合成:
- 5′-O-(4,4′-二甲氧基三苯甲基)-2′-脱氧胸苷 3′-O-(2-氰基乙基 N,N-二异丙基亚磷酰胺)
- 4,5-二(酰胺肟基)咪唑 [4,5-(DAO)Im]
- 1-(4-甲氧基苄基)-4,5-二氰基咪唑
- 新型咪唑并 [4 5-e] [1 3] 二氮杂类似物
- N-苄基-4,5-二氰基咪唑]
訊號詞
Danger
危險聲明
危險分類
Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
閃點(°F)
Not applicable
閃點(°C)
Not applicable
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
4, 5-Dicyanoimidazole.
Sebesta DP and Vagle K.
e-EROS Encyclopedia of Reagents for Organic Synthesis. null
Structural clues to UO₂²⁺/VO₂⁺ competition in seawater extraction using amidoxime-based extractants.
Steven P Kelley et al.
Chemical communications (Cambridge, England), 50(83), 12504-12507 (2014-09-06)
Here we present the first structural comparison of amidoxime complexes of UO2(2+) and VO2(+) (the main competitor in the extraction of uranium from seawater using amidoxime-based sorbents) using a 4,5-di(amidoxime)-functionalized imidazole ligand. The amidoxime groups resist tautomerization in both cases
Synthesis of 1-benzyl-8, 9-dihydroimidazo [4, 5-c] pyrrolo [3, 2-g] quinolin-4 (5H)-one via palladium-catalyzed intramolecular arylation.
Delest B, et al.
Tetrahedron, 60(29), 6079- 6083 (2004)
C Vargeese et al.
Nucleic acids research, 26(4), 1046-1050 (1998-03-21)
A new activator for the coupling of phosphoramidites to the 5'-hydroxyl group during oligonucleotide synthesis is introduced. The observed time to complete coupling is twice as fast with 4, 5-dicyanoimidazole (DCI) as the activator, compared with 1 H -tetrazole. The
Matthew T Holden et al.
Analytical chemistry, 87(22), 11420-11428 (2015-10-24)
The photolithographic fabrication of high-density DNA and RNA arrays on flexible and transparent plastic substrates is reported. The substrates are thin sheets of poly(ethylene terephthalate) (PET) coated with cross-linked polymer multilayers that present hydroxyl groups suitable for conventional phosphoramidite-based nucleic
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门