429066
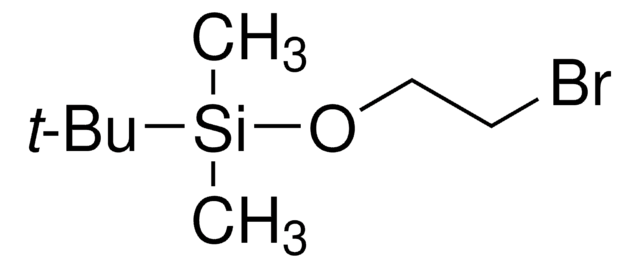
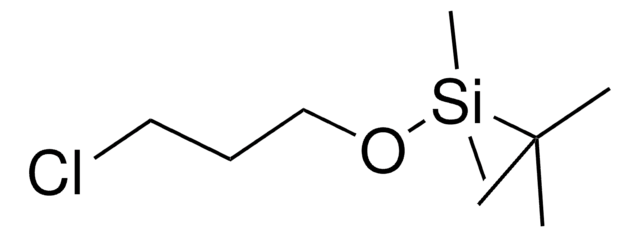
(3-溴丙氧基)-叔丁基二甲基硅烷
97%
别名:
(3-Bromopropoxy)(1,1-dimethylethyl)dimethylsilane, 1-((tert-Butyldimethylsilyl)oxy)-3-bromopropane, 1-Bromo-3-(tert-butyldimethylsiloxy)propane, 1-Bromo-3-[(tert-butyldimethylsilanyl)oxy]propane, 1-Bromo-3-[(tert-butyldimethylsilyl)oxy]propane, 3-((tert-Butyldimethylsilyl)oxy)-1-bromopropane
登录查看公司和协议定价
所有图片(1)
About This Item
线性分子式:
Br(CH2)3OSi(CH3)2C(CH3)3
CAS号:
分子量:
253.25
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
一般說明
(3-Bromopropoxy)-tert-butyldimethylsilane is a bromo silyl ether.
應用
(3-Bromopropoxy)-tert-butyldimethylsilane may be used to introduce propanol functionality to many pharmaceuticals.
It may be used as an alkylating agent in the synthesis of the following:
It may be used as an alkylating agent in the synthesis of the following:
- N-[2-[N-[3-(tert-butyldimethylsilyloxy)propyl]-N-ethylamino]ethyl]phthalimide
- O-(3-tert-butyldimethylsilyloxypropyl)-N-(tert-butoxycarbonyl)-L-tyrosine methyl ester
- tert-butyldimethyl-[3-(3-methyl-2-nitrophenoxy)propoxy]silane
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
標靶器官
Respiratory system
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
185.0 °F - closed cup
閃點(°C)
85 °C - closed cup
其他客户在看
Ramani R Ranatunge et al.
Journal of medicinal chemistry, 47(9), 2180-2193 (2004-04-16)
The synthesis of a series of novel pyrazoles containing a nitrate (ONO(2)) moiety as a nitric oxide (NO)-donor functionality is reported. Their COX-1 and COX-2 inhibitory activities in human whole blood are profiled. Our data demonstrate that pyrazole ring substituents
Synthesis of O-(3-[18F] Fluoropropyl)-L-tyrosine (L-[18F] FPT) and its biological evaluation in 9L tumor bearing rat.
Moon BS, et al.
Bull. Korean Chem. Soc., 26(1), 91-96 (2005)
A concise total synthesis of (+/-)-vigulariol.
J Stephen Clark et al.
Angewandte Chemie (International ed. in English), 46(3), 437-440 (2006-12-06)
Mechanisms of ZnII-activated magnetic resonance imaging agents.
Major JL, et al.
Inorganic Chemistry, 47(22), 10788-10795 (2008)
Margaret M Faul et al.
The Journal of organic chemistry, 69(9), 2967-2975 (2004-04-24)
Synthesis of indolo[6,7-a]pyrrolo[3,4-c]carbazoles 1, a new class of cyclin D1/CDK4 inhibitors, by oxidation of the corresponding aryl indolylmaleimides 2, will be described. Two approaches to the synthesis of 2 were identified that required new methods for the synthesis of 7-substituted
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门