所有图片(1)
About This Item
经验公式(希尔记法):
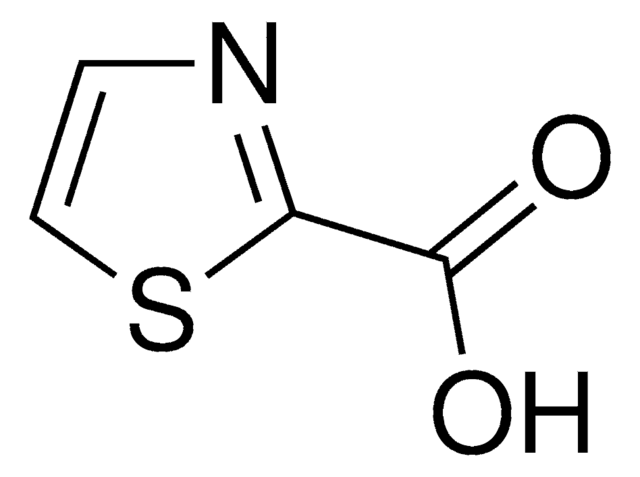
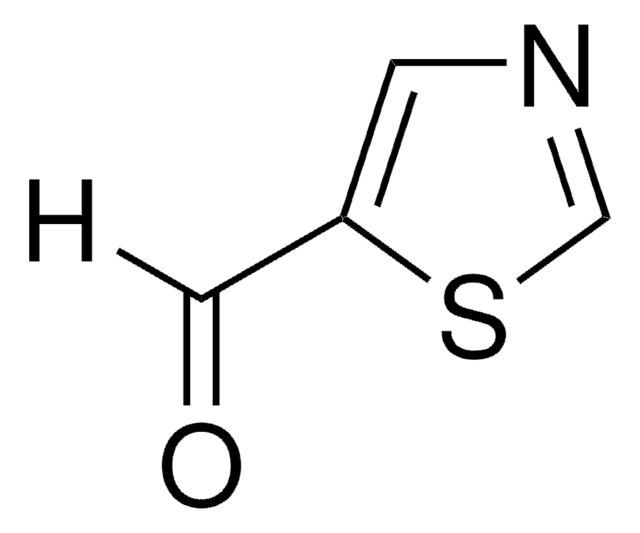
C4H3NOS
CAS号:
分子量:
113.14
MDL號碼:
分類程式碼代碼:
12352100
PubChem物質ID:
NACRES:
NA.22
推荐产品
化驗
97%
形狀
liquid
折射率
n20/D 1.574 (lit.)
bp
61-63 °C/15 mmHg (lit.)
密度
1.288 g/mL at 25 °C (lit.)
儲存溫度
2-8°C
SMILES 字串
O=Cc1nccs1
InChI
1S/C4H3NOS/c6-3-4-5-1-2-7-4/h1-3H
InChI 密鑰
ZGTFNNUASMWGTM-UHFFFAOYSA-N
一般說明
2-Thiazolecarboxaldehyde is a thiazole aldehyde derivative. It undergoes Baylis–Hillman reaction with methyl acrylate catalyzed by DABCO (1,4-diazabicyclo[2.2.2]octane). The reaction mechanism has been studied by electrospray ionization mass spectrometry (ESI-MS).
應用
紫杉烷类似物的有用结构单元。
2-Thiazolecarboxaldehyde may be used as a reactant in the following syntheses:
- Benzothiazine N-acylhydrazones, having potential antinociceptive and anti-inflammatory activity.
- Thiazole-2-yl-(amino)methylphosphonate diethyl esters.
- Imino ester by reacting with L-leucine t-butyl ester hydrochloride.
儲存類別代碼
10 - Combustible liquids
水污染物質分類(WGK)
WGK 3
閃點(°F)
154.4 °F - closed cup
閃點(°C)
68 °C - closed cup
其他客户在看
Online mechanistic investigations of catalyzed reactions by electrospray ionization mass spectrometry: a tool to intercept transient species in solution.
Santos LS.
European Journal of Organic Chemistry, 2008(2), 235-253 (2008)
The Morita-Baylis-Hillman Reaction: Advances and Contributions from Brazilian Chemistry.
Santos MS, et al.
Current Organic Synthesis, 12(6), 830-852 (2015)
Yasuyuki Takeda et al.
Bioorganic & medicinal chemistry letters, 14(12), 3209-3215 (2004-05-20)
To improve the metabolic stability of 3, which exhibited both in vitro antitumor activity and in vivo efficacy by both iv and po administration, we designed and synthesized new taxane analogues. Most of the synthetic compounds maintained excellent antitumor activity
Synthesis of new thiazole-2,-4, and-5-yl-(amino) methylphosphonates and phosphinates: unprecedented cleavage of thiazole-2 derivatives under acidic conditions.
Olszewski TK and Boduszek B.
Tetrahedron, 66(45), 8661-8866 (2010)
Armel A Agbodjan et al.
The Journal of organic chemistry, 73(8), 3094-3102 (2008-03-25)
A practical asymmetric synthesis of a highly substituted N-acylpyrrolidine on multi-kilogram scale is described. The key step in the construction of the three stereocenters is a [3+2] cycloaddition of methyl acrylate and an imino ester prepared from l-leucine t-butyl ester
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门





![苯并[b]噻吩-2-甲醛 97%](/deepweb/assets/sigmaaldrich/product/structures/321/060/32405a4e-5720-4c6d-91cf-115c747270c4/640/32405a4e-5720-4c6d-91cf-115c747270c4.png)