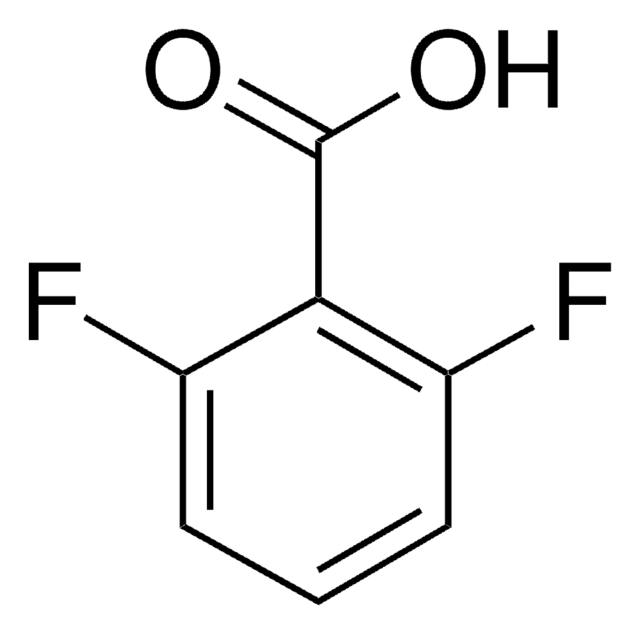
推荐产品
化驗
97%
mp
122-125 °C (lit.)
SMILES 字串
OC(=O)c1ccccc1F
InChI
1S/C7H5FO2/c8-6-4-2-1-3-5(6)7(9)10/h1-4H,(H,9,10)
InChI 密鑰
NSTREUWFTAOOKS-UHFFFAOYSA-N
正在寻找类似产品? 访问 产品对比指南
應用
2-氟苯甲酸可用于制备Zaragozic酸A类似物。
訊號詞
Warning
危險聲明
危險分類
Eye Irrit. 2 - Skin Irrit. 2
儲存類別代碼
11 - Combustible Solids
水污染物質分類(WGK)
WGK 3
個人防護裝備
dust mask type N95 (US), Eyeshields, Gloves
其他客户在看
J Toretsky et al.
Nuclear medicine and biology, 31(6), 747-752 (2004-07-13)
The clinical response to antitumor therapy is measured using imaging, such as CT or MRI, 6-12 weeks following chemotherapy treatment. The images at that time reflect both tumor cell death and new growth. Therefore, the amount of tumor cell death
S Y Lee et al.
Nuclear medicine and biology, 28(4), 391-395 (2001-06-08)
In vitro metabolism of acetylcholinesterase inhibitors containing 3-[(18)F]fluoromethylbenzyl- ([(18)F]1) and 4-[(18)F]fluorobenzyl-piperidine moieties ([(18)F]2) was studied and compared with the in vivo metabolism. Defluorination of the [(18)F]1 mainly occurred to generate [(18)F]fluoride ion both in vitro and in vivo. In contrast
U Schennen et al.
Journal of bacteriology, 161(1), 321-325 (1985-01-01)
Three strains of anaerobically benzoate-degrading, denitrifying bacteria of the genus Pseudomonas were able to grow on 2-fluorobenzoate as the sole carbon and energy source. Fluoride ion release was stoichiometric, and the reduction of dissolved organic carbon indicated total degradation. Cells
Microbial degradation of synthetic organochlorine compounds.
K Motosugi et al.
Experientia, 39(11), 1214-1220 (1983-11-15)
C J Springer et al.
Journal of medicinal chemistry, 37(15), 2361-2370 (1994-07-22)
The synthesis of six novel fluorinated potential prodrugs for antibody-directed enzyme prodrug therapy is described. The [2- and 3-fluoro-4-[bis(2-chloroethyl)amino]benzoyl]-L-glutamic acid (9 and 21), their bis(mesyloxy)ethyl derivatives (7 and 19), and their chloroethyl (mesyloxy)-ethyl derivatives (8 and 20) are bifunctional alkylating
我们的科学家团队拥有各种研究领域经验,包括生命科学、材料科学、化学合成、色谱、分析及许多其他领域.
联系技术服务部门